Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
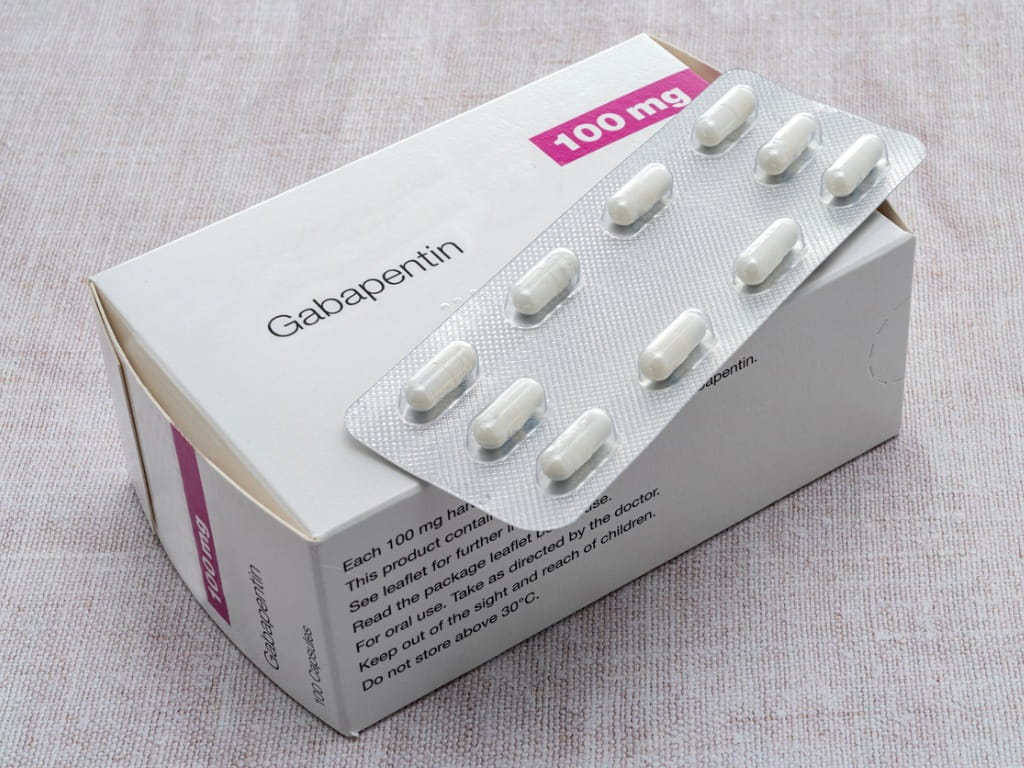
GABAPENTIN (GA ba pen tin) treats nerve pain. It works by calming overactive nerves in your body. Gabapentin enacarbil extended release tablets are available under the trade name Horizant: -The recommended dosage is 600 mg orally 2 times a day. Therapy should be initiated at a dose of 600 mg orally in the morning for 3 days of therapy, then increased to 600 mg 2 times a day (1200 mg/day) on day four. Gabapentin enacarbil extended release tablets available under the trade name Horizant and gabapentin are not interchangeable. Use: Postherpetic neuralgia. Usual Adult Dose of Horizant for Restless Legs Syndrome: 600 mg orally once daily with food at about 5 PM. A dose of 1,200 mg once daily provided no additional benefit compared with the 600 This medication is used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. This condition is called postherpetic neuralgia. Gabapentin belongs to a class of drugs known as antiseizure drugs (also called anticonvulsant or antiepileptic drugs). Horizant™ will cost approximately $120 per month 7 as compared with generic gabapentin 600 mg tablets which cost approximately $31.00 per month 8 It is unclear how gabapentin enacarbil will compare with Gralise, an alternate gabapentin extended release formulation that was recently approved for the treatment of post-herpetic neuralgia. 48 HORIZANT Extended-Release Tablets, 300 mg, are red, oval-shaped tablets debossed 49 with “GS TF7” and 600 mg, are white to off-white, oval-shaped tablets debossed with 50 “GS LFG”. Both the 300 mg and 600 mg tablets may contain occasional black/grey spots. 51 A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose and caused an increase in adverse reactions. Patients with creatinine clearance <60 mL/min have a different dosing regimen. 1 † The molecular weight of Horizant® is ~2 times that of gabapentin: Two 600 mg doses of Horizant® contain ~626 mg of gabapentin. 3 *The dose is normalized to 1,000 mg equivalent (mg-eq) of gabapentin. t max is presented as median (minimum, maximum). b.i.d. = twice daily; q.d. = once daily; t.i.d. = 3 times daily; C max = maximum plasma concentration over the last dosing day; t max = time to reach maximum concentration over the dosing interval in which the C max was observed (if the maximum value occurred more than once in Detailed Gabapentin dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments. Find patient medical information for Horizant (gabapentin enacarbil) on WebMD including its uses, side effects and safety, interactions, pictures, warnings, and user ratings Indication: Gabapentin enacarbil is a novel transported pro-drug of gabapentin designed to overcome the pharmacokinetic limitations of gabapentin and is being developed at a 600 mg oral dose for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS). Presentation: HorizantTM (gabapentin enacarbil) Extended Release Tablets are When Horizant is taken for postherpetic neuralgia, the typical starting dose is 600 mg every morning with food for 3 days. Then, the dose is usually increased to 600 mg twice daily with food. If Horizant is prescribed for RLS, the usual dose is 600 mg once daily at around 5PM with food. Gralise HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the management of postherpetic neuralgia (PHN Each HORIZANT Extended-Release Tablet contains 300 mg or 600 mg of gabapentin enacarbil and the following inactive ingredients: colloidal silicon dioxide, dibasic calcium phosphate dihydrate, glyceryl behenate, magnesium stearate, sodium lauryl sulfate, and talc. The IASP guidelines recommend both immediate- and extended-release gabapentin IASP [Finnerup 2015]. In contrast, a guideline from the American Academy of Neurology Chen C, Cowles VE, Hou E. Pharmacokinetics of gabapentin in a novel gastric-retentive extended-release formulation: comparison with an immediate-release formulation and effect of dose escalation and food. J Clin Pharmacol. 2011;51(3):346-358.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |