Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
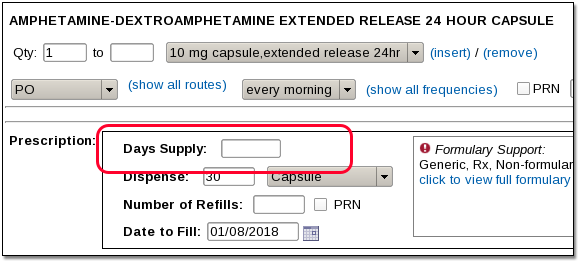
Review Ohio BON rules for use of OARRS and requirements for opioid prescriptions. Discuss updates to law and rules governing medication assisted treatment (MAT) in Ohio. However, due to a spike in gabapentin-related fatalities, Ohio, Kentucky and West Virginia have moved to list the drug as a controlled substance at the state level. Other states are recognizing the growing abuse problem with gabapentin and have, at the very least, mandated that it be included in their prescription drug monitoring programs. Gabapentin, an opioid alternative, is being abused at an alarming rate across Ohio. As a result, state officials are considering reclassifying this drug as a controlled substance. Gabapentin (Neurontin) is typically prescribed to treat seizures and nerve pain in adults. Ohio Administrative Code / 4731 / Chapter 4731-11 | Controlled Substances . Effective: July 31, 2023 sedative hypnotic drug, carisprodol, tramadol, or gabapentin C.S.A. Controlled Substance Act *These are drug products which: (1) may be dispensed only upon a prescription issued by a practitioner and, (2) contain controlled substances but have been specifically excepted from the controlled substances schedules. (Title 21, CFR 1308.31.) Accordingly, these drugs are legally classified as dangerous drugs in What Is Gabapentin and How Does It Work? Gabapentin, brand-name Neurontin (among others), is an anticonvulsant medication approved by the U.S. Food and Drug Administration (FDA) to treat certain seizure disorders and postherpetic neuralgia (a type of nerve pain associated with shingles). Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Gabapentin has not been reclassified as a controlled substance, but it is being added to the Board’s list of drugs reportable to OARRS following increased reports of misuse, abuse, and concomitant abuse of gabapentin nationwide.1. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Stats PDMP Interactive Data Tool. Ohio's prescription drug monitoring program, known as the Ohio Automated Rx Reporting System (OARRS), collects information on the distribution of prescription controlled substances and two non-controlled drugs, gabapentin and naltrexone, to Ohio patients. Established in 2006, OARRS collects information on all outpatient prescriptions for controlled substances and two non-controlled substances (gabapentin & naltrexone) dispensed by Ohio-licensed pharmacies and personally furnished by Ohio prescribers. This data is reported every 24 hours and is maintained in a secure database. Gabapentin’s unscheduled status reflects its lower potential for abuse or dependency compared to controlled substances. However, the FDA monitors gabapentin for potential misuse, particularly when combined with other central nervous system depressants. This oversight aims to balance its therapeutic benefits against abuse risks. Regional Variation 77 South High Street, 17th Floor, Columbus, Ohio 43215 T: (614) 466.4143 | F: (614) 752.4836 | contact@pharmacy.ohio.gov | www. pharmacy.ohio.gov. Controlled Substance Reference Table Annual Review Completed for all Drug Entries on 9-15-2019 . Please be advised that the information contained in this table is compiled solely registration numbers for gabapentin prescriptions issued by veterinarians. As a reminder, gabapentin is not a controlled substance in Ohio and so a veterinarian is not required to have a DEA registration number to prescribe the medication. Additionally, veterinarians do not have NPI numbers. Gabapentin, a drug viewed as an alternative to opioids, is being abused across Ohio, experts and state officials warn. The misuse could lead the state to reclassify the drug. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. on all outpatient prescriptions for controlled substances and two non-controlled substances (gabapentin and naltrexone) dispensed by Ohio-licensed pharmacies and personally furnished by Ohio prescribers. This data is reported every 24 hours and is maintained in a secure database. Drug wholesalers and manufacturers are also required to submit care, the Ohio Board of Pharmacy created Ohio’s Prescription Drug Monitoring Program (PDMP), known as the Ohio Automated Rx Reporting System (OARRS). OARRS collects information on all outpatient prescriptions for controlled substances and gabapentin dispensed by Ohio-licensed pharmacies and personally furnished by licensed prescribers in Ohio.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |