Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
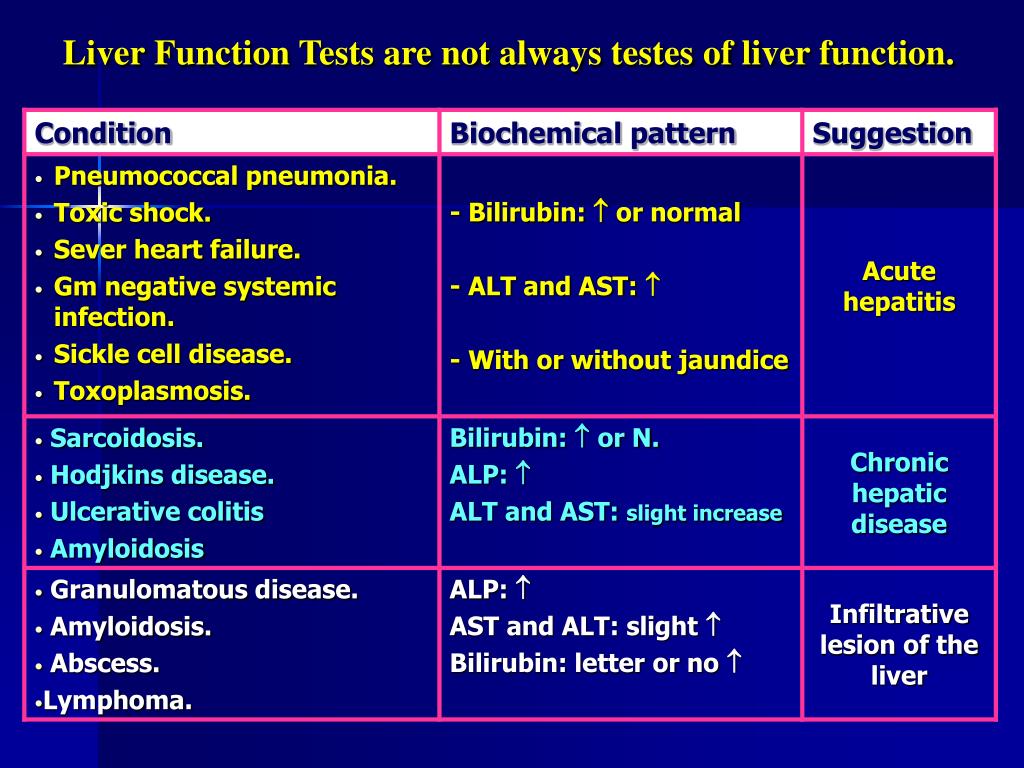
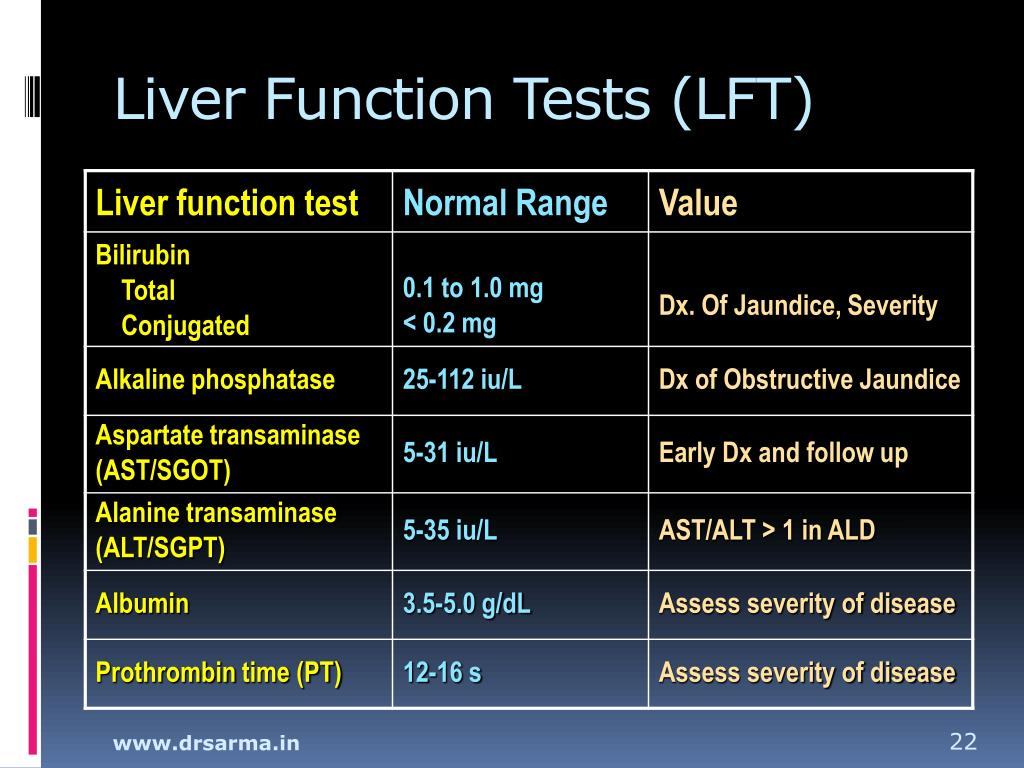
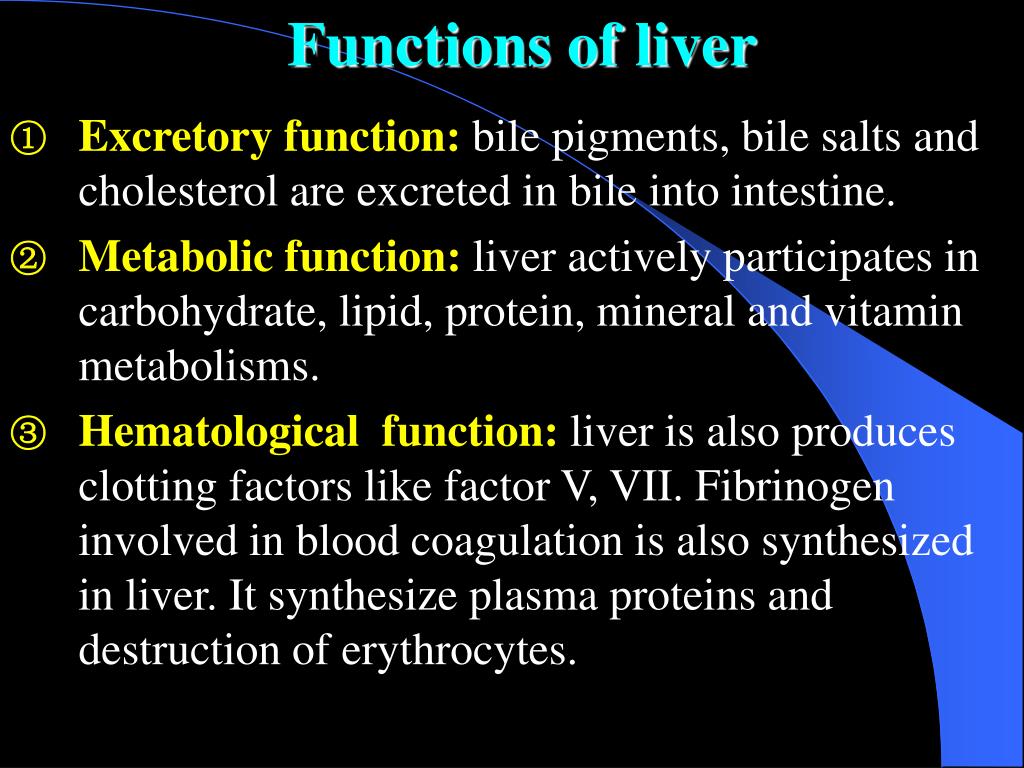
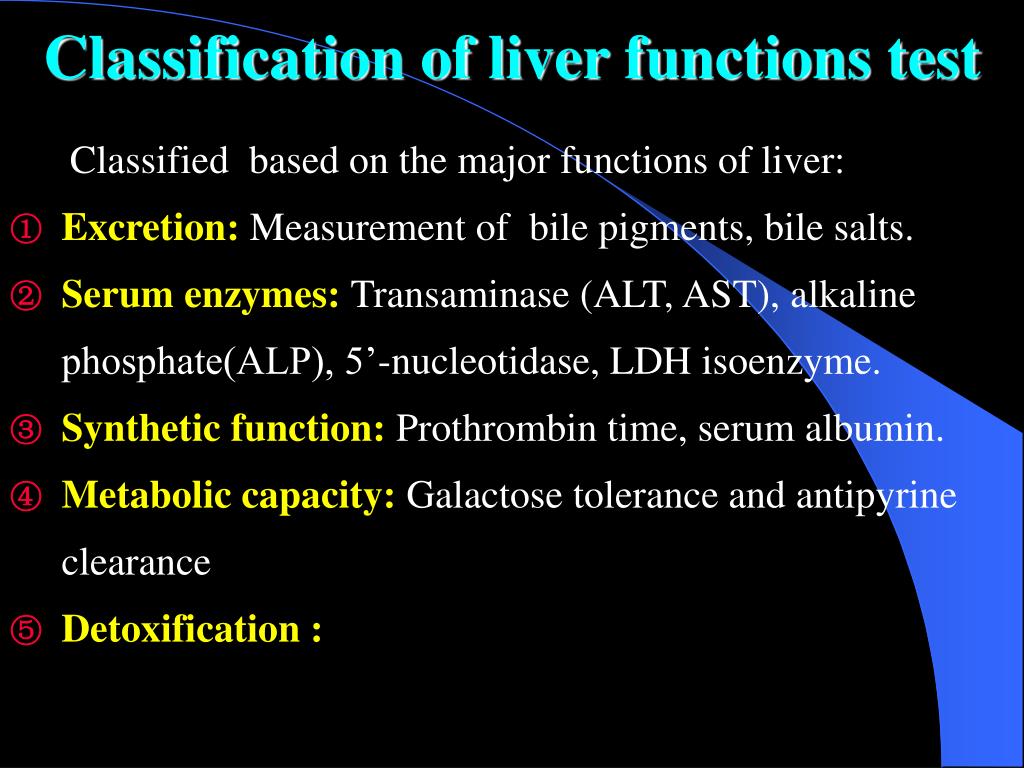
Our previous and several other studies shows that long term treatment with old or new anti epileptic drugs affect liver function from transient state to a fatal liver damage (1,8-10). But when considered GPN such an effect is quite less and no report of death or fatal liver damage. The discontinuation of gabapentin leads to an improvement in liver test abnormalities. The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. (Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]). Can administration of gabapentin for trigeminal neuralgia dramatically increase liver function tests? Gabapentin induced cholestasis was thought likely and the drug was stopped. After this, clinical symptoms and liver function tests improved gradually (figure). A liver biopsy showed normal liver architecture with evidence of portal tract expansion by a chronic inflammatory cell infiltrate that incorporated eosinophils and neutrophils. Drug manufacturer Pfizer classifies abnormal liver function tests as an infrequent side effect for gabapentin 5. There is insufficient data to estimate incidence for these or establish whether gabapentin is the sole cause of elevated liver function tests, notes Pfizer. Does gabapentin affect liver function tests? Gabapentin is excreted unchanged in the urine. It does not affect the liver enzymes and has not been associated with hepatotoxicity. Patients with liver disease may require long-term AED treatment. The selection of AEDs in these individuals deserves careful consideration. Drug metabolism depends on the integrity of the hepatocyte, the blood flow, and the patency of the hepatobiliary system. 5 Because of the large hepatic reserve, the liver dysfunction must be severe to cause substantial alterations in drug metabolism. 27 Gabapentin enacarbil and gabapentin are associated with a low rate of transient serum enzyme elevations during treatment and with rare instances of clinically apparent liver injury. Gabapentin enacarbil is a long acting form of gabapentin that is used for restless leg syndrome and for painful postherpetic neuropathy. Liver function tests at this stage showed aspartate transaminase 104 U/l (reference range 10-40 U/l), bilirubin 199 μmol/l (5-20 μmol/l), alkaline phosphatase 210 U/l (25-115 U/l), and γ-glutamyltransferase 839 U/l (<85 U/l). Prior records revealed completely normal liver function tests 6 months prior to current presentation. Ultrasound of the right upper quadrant demonstrated small volume of ascites but was otherwise unremarkable with no abnormality of hepatic parenchyma or biliary ducts. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported. When your liver is damaged, these enzymes leak out into your blood and can be measured with blood testing called liver function testing. There are several liver enzymes, but the ones that show liver damage from medications are aspartate transaminase (AST) and alanine transaminase (ALT). Gabapentin is an anticonvulsant medication commonly used to treat epilepsy and neuropathic pain. Rare cases of liver and kidney damage have been reported with Gabapentin use. Individuals with pre-existing liver or kidney conditions may be at a higher risk. Regular monitoring of liver and kidney function is essential while taking Gabapentin. In most cases, gabapentin doesn’t hurt the liver or kidneys, though proper dosing is important to prevent side effects. Learn how gabapentin affects the liver and kidneys here. Lab work revealed abnormal liver function tests, with ALP 1,232 IU/L, AST 291 IU/L, and ALT 188 IU/L, and normal total bilirubin of .8 mg/dL. Prior records revealed completely normal liver function tests 6 months prior to presentation. A 47-year-old Caucasian woman (Supplemental Table 3, patient # 16) who received 50 mg lamotrigine for 437 days presented with hepatocellular liver injury (Peak ALT 798 U/L, Alk P 664 U/L, and total bilirubin 9.8 mg/dL) had a liver biopsy during the acute liver injury episode which showed cholestatic liver injury, with severe duct injury, focal Changes in liver function may be attributed to free radical damage induced by gabapentin, as documented in this study, where the drug enhanced antioxidant defense systems and elevated liver NO
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |