Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
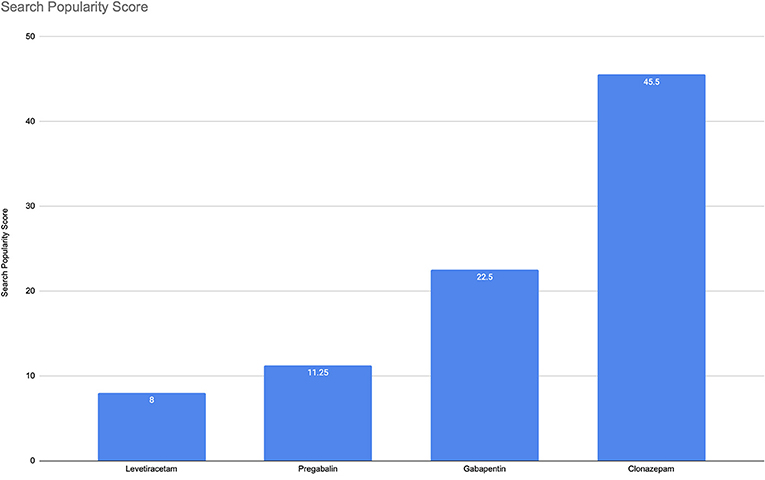
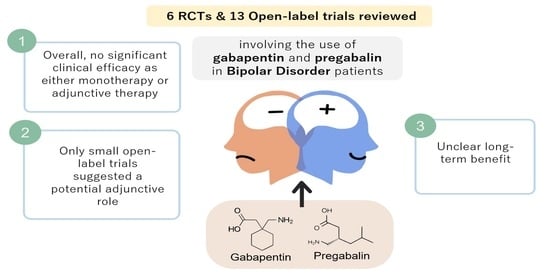
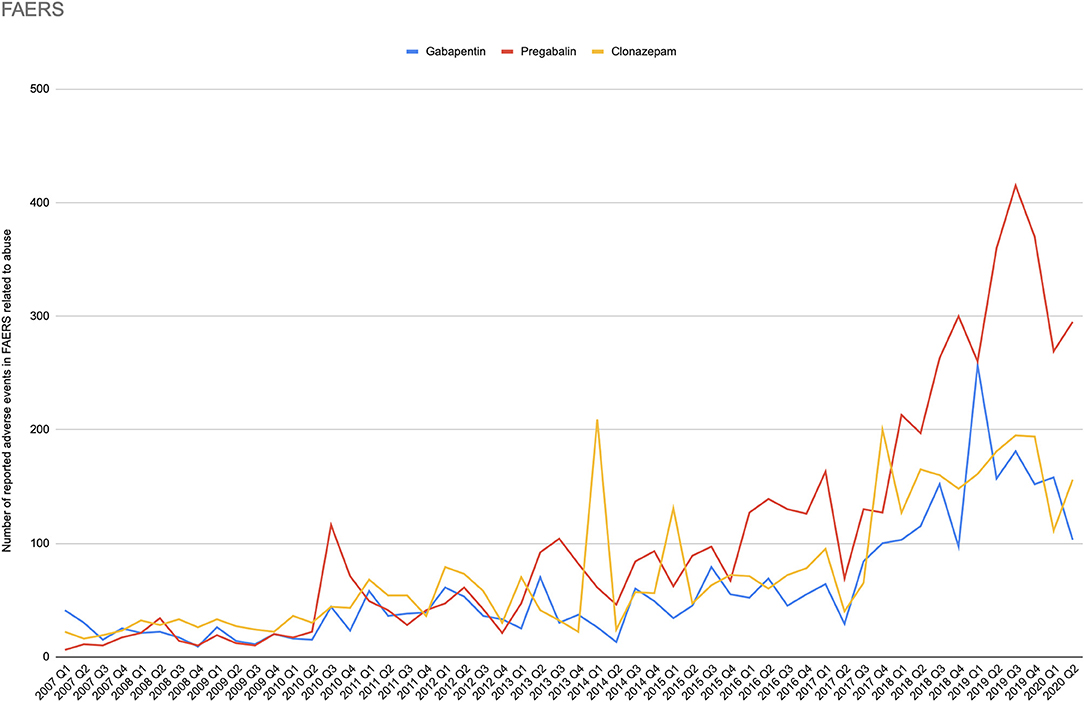
Evidence suggests gabapentinoids possess potential for abuse, particularly in individuals with a history of opioid abuse, and reports of such abuse are increasingly being documented. Prescribers should be aware of high-risk populations and monitor for signs of abuse. This review summarizes current evidence on the abuse and misuse of the gabapentinoids pregabalin and gabapentin. Pharmacovigilance studies, register-based studies, surveys, clinical toxicology studies, and forensic toxicology studies were identified and scrutinized with the goal to define the problem, identify risk factors, and discuss possible methods to reduce the potential for abuse and Gabapentin and pregabalin are indicated for the treatment of neuropathic pain only. Use in other types of pain is unapproved. Cases of abuse and dependence have been reported with gabapentin and pregabalin. Evaluate patients for a history of substance abuse and observe for signs of misuse or abuse. This study interestingly found that gabapentin and pregabalin had many similar most frequently reported adverse effects, including drug ineffectiveness, pain, nausea, fatigue, and dizziness. 22.93% of all gabapentin reports and 26.13% of all pregabalin reports to FAERS involved at least one abuse-related adverse event. Based on extensive literature search, this is the first study investigating the abuse potential of pregabalin and gabapentin using two different pharmacovigilance methods: disproportionality analysis in the FAERS and Google search analytics. Evidence suggests that gabapentinoid misuse/abuse represents a growing trend that is causing significant patient harm. Prescribers should exercise appropriate caution with use in high-risk populations and monitor for signs of misuse or abuse. The PRR of pregabalin versus gabapentin abuse-related events was 1.77. Conclusion: Though not traditionally thought of as drugs of abuse, over 600 cases of gabapentinoid abuse were reported in the time frame analyzed, prompting the need for further study and regulatory investigation. As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Epidemiological and case report evidence suggests that the antiepileptic and analgesic medication gabapentin is being misused internationally at a rate of about 1%, with substance abuse populations at special risk for misuse/abuse. Keywords: gabapentin, prescription drug misuse, systematic review, diversion, substance abuse. Gabapentinoid (pregabalin and gabapentin) abuse is increasingly being reported. To assess the extent of gabapentinoid abuse, characteristics of typical abusers, patterns of abuse, and potential harms in order to bring this trend to providers’ attention. In Europe, in mid-2011, about 30 cases of dependence, abuse or withdrawal symptoms attributed to pregabalin had been reported to Swedish and French pharmacovigilance centres and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). About 20 cases of gabapentin addiction were publishe Available evidence also suggests that abuse and misuse are more frequent in users of pregabalin compared with users of gabapentin. Health professionals and prescribers should be aware of the risk for misuse of pregabalin and gabapentin, which eventually could lead to abuse, substance dependence, and intoxications. Health professionals and prescribers should be aware of the risk for misuse of pregabalin and gabapentin, which eventually could lead to abuse, substance dependence, and intoxications. In the last ten years, gabapentin and pregabalin have been becoming dispensed broadly and sold on black markets, thereby, exposing millions to potential side-effects. Meanwhile, several pharmacovigilance-databases have warned for potential abuse liabilities and overdose fatalities in association wit The gabapentinoid group consists of gabapentin and pregabalin. Structurally similar to the inhibitory neurotransmitter GABA (gamma-aminobutyric acid), gabapentinoids bind to a subunit (protein α2-δ) of a voltage-dependent calcium channel in the central nervous system, decreasing the intracellular calcium influx induced by cell depolarization, and the release of exciting neurotransmitters. Evidence suggests that gabapentinoid misuse/abuse represents a growing trend that is causing significant patient harm. Prescribers should exercise appropriate caution with use in high-risk populations and monitor for signs of misuse or abuse. Physicians considering prescribing gabapentinoids for neurological/psychiatric disorders should carefully evaluate a possible previous history of drug abuse, whilst being able to promptly identify signs of pregabalin/gabapentin misuse and provide possible assistance in tapering off the medication. Despite their inherent abuse potential, gabapentinoids (gabapentin and pregabalin) may be safer than presumed and offer prescribers an effective opioid-alternative treatment for certain types of neuropathic pain. positive addiction history and drug abuse in 48.1% of pregabalin and 18.6% of gabapentin cases; 91.4 of pregabalin abusers and 87.5 of gabapentin abusers had concomitant opioid use, other psychoactive substances were found in the remaining cases Misuse of the γ-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. British Journal of Clinical Pharmacology. 78(1):190–191. [PMC free article] [Google Scholar] Millar J, Sadasivan S, Weatherup N, & Lutton S 2013. Lyrica nights - recreational pregabalin abuse in an urban emergency department.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |