Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 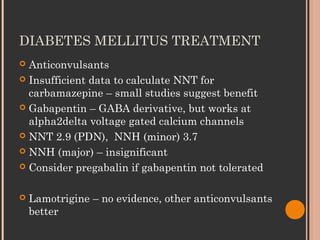 |
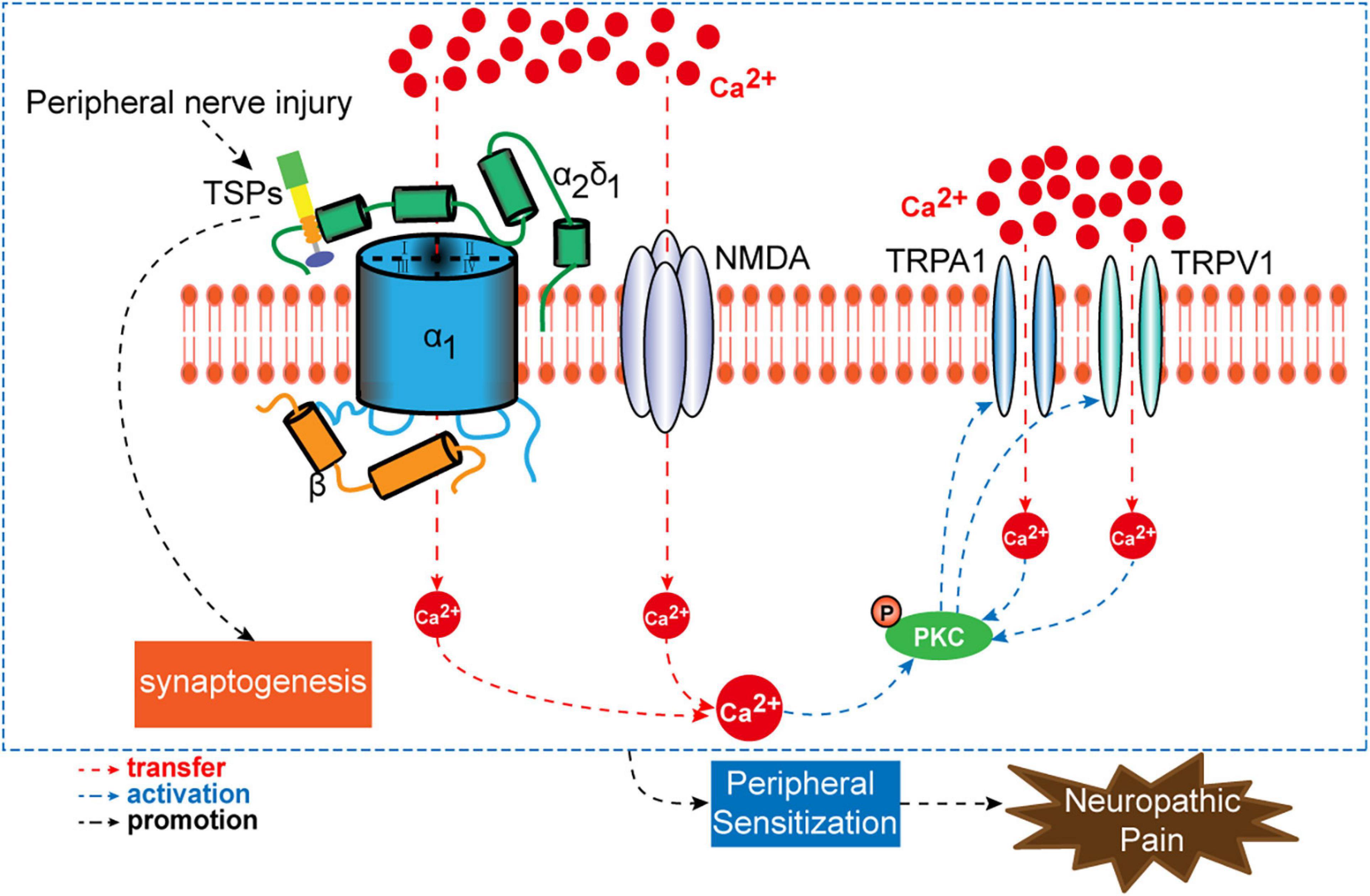
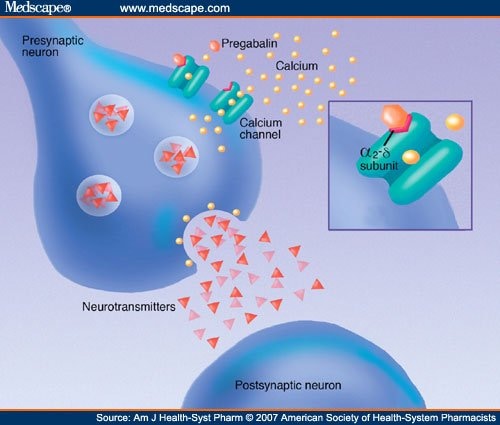
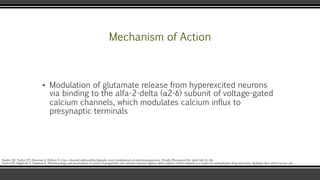
Pregabalin is structurally related to the antiepileptic drug gabapentin and the site of action of both drugs is similar, the alpha2-delta (alpha2-delta) protein, an auxiliary subunit of voltage-gated calcium channels. Pregabalin subtly reduces the synaptic release of several neurotransmitters, apparently by binding to alpha2-delta subunits, and GBP and PGB exert their analgesic actions by selectively binding the α 2 δ 1 auxiliary subunit of voltage-sensitive calcium channels, thereby inhibiting channel function. Numerous tissues express the α 2 δ 1 subunit where GBP and PGB can alter calcium-mediated signaling events. Pregabalin is structurally related to the antiepileptic drug gabapentin and the site of action of both drugs is similar, the alpha 2 –delta (α 2 –δ) protein, an auxiliary subunit of voltage-gated calcium channels. During the opioid epidemic, there has been a significant increase in the number of prescriptions for gabapentinoids (gabapentin and pregabalin), which block α 2 δ-subunit of voltage-gated calcium channels (α 2 δ-VGCCs). Gabapentinoids are commonly used in combination with opioids and frequently detected in drug overdose victims. Several in vitro studies indicate that gabapentin and pregabalin reduce the artificially stimulated influx of calcium via presynaptic voltage-gated calcium channels and consequently reduce the stimulated release of synaptic neurotransmitters (reviewed [14]). Interesting review discussing the interaction of a2d-1 with N-methyl-D-aspartate–sensitive glutamate receptors, neurexin1a, thrombospondins, and other presynaptic proteins, in addition to actions at calcium channels, and the importance of these findings for gabapentin and pregabalin therapeutic effects. Pregabalin is similar in structure to gabapentin, and both gabapentin and pregabalin bind selectively with high affinity to the α 2 δ subunit of voltage-gated calcium channels, and reduce the release of neurotransmitters evoked by synchronous stimulation of tissue slices from the spinal cord and brain, presumably as a result of binding to the By purifying the protein to which gabapentin bound, and determining its identity as the α2δ1 subunit of voltage gated calcium channels, it was possible to make progress in developing new compounds with similar activities to gabapentin, including pregabalin. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel alpha2-delta [Cavalpha2-delta] ligands To the Editor: Gabapentinoids, including gabapentin and pregabalin, bind to the α2δ1 subunit of calcium channel (α2δ1) to relieve neuropathic pain 1.Although gabapentinoids are among the most effective medications to treat chronic neuropathic pain 2, their use in managing postoperative acute pain has been controversial. α 2 δ Auxiliary Calcium Channel Subunits. Originally designed as analogs of GABA (Fig. 1), neither gabapentin nor pregabalin has any significant agonist-like effect on GABA A or GABA B receptors, nor obvious effects on levels of GABA (Lanneau et al. 2001; Jensen et al. 2002). 6 Pregabalin is six-times more potent than gabapentin in binding affinity to the alpha 2-delta voltage-gated calcium channel. 9 The manufacturer states that 50 mg of pregabalin is approximately equal to 300 mg of gabapentin. This alteration of calcium channel function is not to be confused with calcium-channel blockers. Pregabalin and Interesting review discussing the interaction of a2d-1 with N-methyl-D-aspartate–sensitive glutamate receptors, neurexin1a, thrombospondins, and other presynaptic proteins, in addition to actions at calcium channels, and the importance of these findings for gabapentin and pregabalin therapeutic effects. Structures of amino acid drugs and calcium channel subunits. (A) Chemical structures (derived from X-ray crystal analysis) of GABA, pregabalin and gabapentin show that GABA (the major rapid Pharmacology and Protein Interactions of Pregabalin Pregabalin Is a High-Affinity α2δ Subunit Ligand. The alpha-2-delta (α2δ) subunits were first described as auxiliary subunits of the high voltage-activated calcium channels and are encoded by four genes: α2δ1, α2δ-2, α2δ-3 and α2δ-4. α2δ-1-3 are expressed in neurons as well as heart and skeletal muscle tissue (Gong et al., 2001 The depolarizing effect of activating pre‐synaptic 5‐HT 3 Rs could consequently alter the kinetics and/or cycling of calcium channels and create the required conditions for gabapentin to inhibit calcium currents and transmitter release (Suzuki et al. 2005). Rather than their intended pharmacological action on GABA neurotransmission, instead, they exhibit a high affinity for the α2δ-1 and α2δ-2 subunits of voltage-activated calcium channels, wherein binding of gabapentinoids inhibits cellular calcium influx and attenuates neurotransmission. Both gabapentin and the related gabapentinoid pregabalin (Fig. 1a) are approved for use in humans to treat pain, and both molecules are known to interact physically with the Ca v α 2 δ-1 and Ca Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K +-evoked [3 H]-norepinephrine release from rat neocortical slices The gabapentinoid drugs gabapentin and pregabalin are key front-line therapies for various neuropathies of peripheral and central origin. Originally designed as analogs of GABA, the gabapentinoids bind to the α 2 δ-1 and α 2 δ-2 auxiliary subunits of calcium channels, though only the former has been
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |