Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
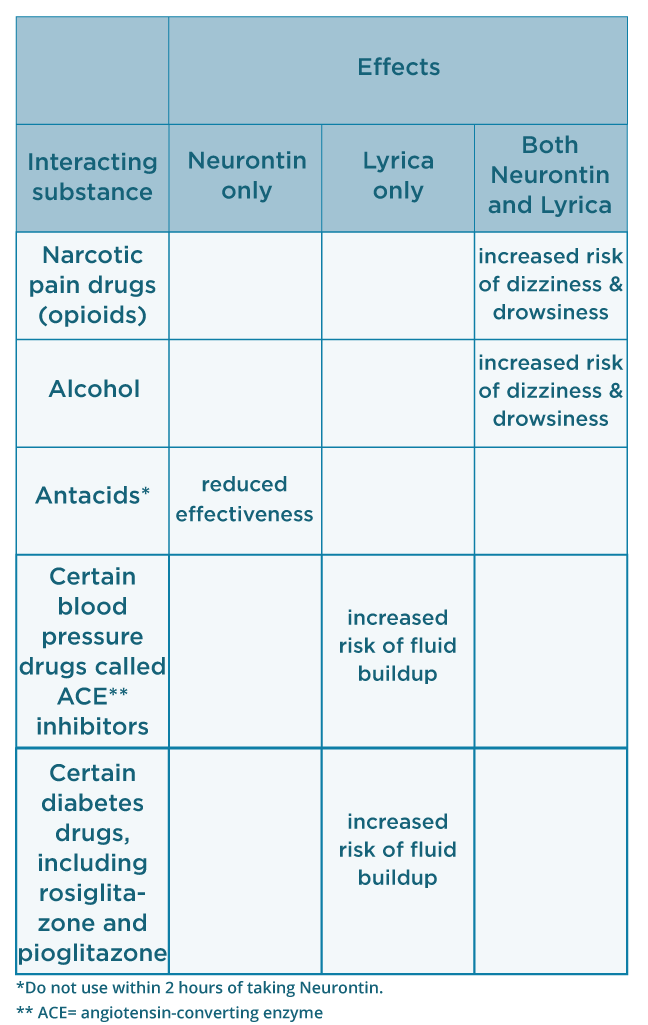 |  |
Pregabalin and gabapentin are both used to treat nerve pain, seizures, and anxiety, but pregabalin has faster absorption, higher bioavailability, and requires lower doses for similar effects compared to gabapentin. Gabapentin is indicated as adjunct therapy for partial seizures and postherpetic neuralgia. 4 Pregabalin is indicated for the same uses as gabapentin, plus the management of fibromyalgia and neuropathic pain associated with diabetes, specifically diabetic neuropathy. 5 What are pregabalin and gabapentin? Pregabalin and gabapentin, collectively gabapentinoids, are primarily anticonvulsant drugs. Over the past decade, they have been increasingly prescribed for pain. 1 They are recommended for neuropathic pain in adults 2 3 (table 1), but are commonly used off-label for other pain disorders such as low back pain, sciatica, and migraine. 9 10 Pregabalin was one While gabapentin (Neurontin) and pregabalin (Lyrica) share many similarities, there are a few things that set them apart. We’ll highlight seven key differences between these medications below. 1. Pregabalin is FDA approved for more uses than gabapentin, but both are often used off-label. Gabapentin is an anticonvulsant with pain-relieving effects that may be used to treat certain seizure disorders or relieve nerve pain. Common side effects include dizziness or drowsiness and it may more. Pregabalin is used in the treatment of nerve pain and also to prevent seizures. The law change means that it will be illegal to possess Gabapentin and pregabalin without a prescription and to supply or sell it to others. What are gabapentin and pregabalin? Gabapentin and pregabalin are drugs used to treat pain. They come in multiple forms including tablet, oral solution and capsules. Find out more about managing pain in MS Gabapentin and pregabalin belong to the same class of medicines — gabapetinoids. So, there are a lot of similarities between them. They both work in a similar way, although researchers aren't Gabapentin and pregabalin are both used to treat partial-onset seizures and nerve pain from shingles (postherpetic neuralgia). Additionally, gabapentin and pregabalin are used off-label to treat a variety of mental health and pain disorders. Pregabalin and gabapentin can both provide relief from pain and be effective ways to manage seizure disorders. However, it’s important to consider the differences between them. Pregabalin is more rapidly absorbed compared to gabapentin, which has a slower absorption rate. Keywords: Gabapentin, pregabalin, pain management, adverse effects, pharmacology. Introduction. The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. The The main difference between gabapentin and pregabalin is their pharmacokinetics. Pregabalin has advantages over gabapentin because it is absorbed more efficiently and has a linear dose-response relationship, meaning a single dose achieves predictable pain reduction. Gabapentin and pregabalin both work to increase the amount of GABA (a pain-fighting transmitter) in your central nervous system. These two medications are in the same drug class. They are gabapentinoids or more generally called anticonvulsants. However, there are differences between them. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). In April 2019, [59] the United Kingdom scheduled gabapentin and pregabalin as Class C drugs under the Misuse of Drugs Act 1971, and as Schedule 3 under the Misuse of Drugs Regulations 2001. [60] However, it is not a controlled substance in Canada, or Australia, and the other gabapentinoids, including phenibut, are not controlled substances From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Gabapentin and pregabalin are similar drugs but differ in several distinct ways. The main differences are their indications—specific uses that the Food and Drug Administration (FDA) has approved them to treat—and their dosages. The Misuse of Drugs Act 1971 (Amendment) Order 2018 classifies pregabalin and gabapentin as Class C drugs under paragraph 1 (b) of Part 3 of Schedule 2 to the Misuse of Drugs Act 1971 (“the 1971 Gabapentin is not the same as pregabalin, even though they both belong to the same class of medicine, called gabapentinoids, and work similarly. Lyrica and Lyrica CR are the only brands of pregabalin. Neurontin is a brand name for gabapentin. Other brands of gabapentin include Gralise and Horizant. Gabapentin and pregabalin are members of a class of anti-convulsive and anti-epileptic drugs called gabapentinoids. Gabapentin was first approved in 1993 and pregabalin followed in 2004. They’ve been widely prescribed to treat certain types of pain as well. Conditions gabapentin and pregabalin are approved to treat include:
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |