Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
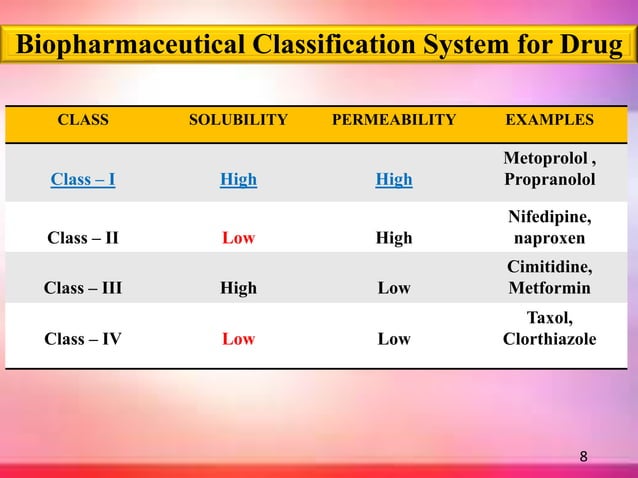
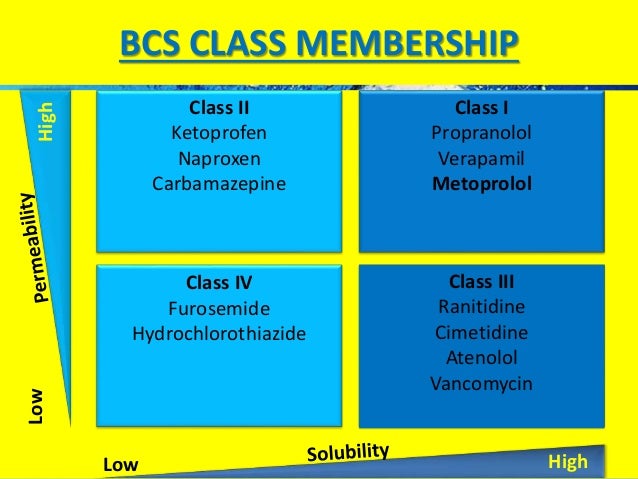
According to the biopharmaceutical classification system (BCS), Gabapentin is considered a class III drug. Its solubility is independent on the pH 21 . Its absorption mainly occurs in the Dezani et al. Equilibrium solubility versus intrinsic dissolution: characterization of lamivudine, stavudine and zidovudine for BCS classification. Braz J Pharm Sci. 2013; 49:853-863. In the BCS system, levetiracetam, gabapentin, and vigabatrin are classified as BCS class 1 drugs . These compounds are completely absorbed, with the exception of gabapentin that is about 70% absorbed in humans ( 40 ), although quite slowly. Pregabalin has been determined to be a Biopharmaceutical Classification System (BCS) Class 1 compound with high solubility/high permeability characteristics. The biowaiver request for this oral 2. BCS Classification The BCS classifies drugs into four categories: 2.1. Examples of Drugs in Each BCS Class 2.1.1. BCS Class I: High Solubility, High Permeability Drugs that dissolve readily in aqueous media and are easily absorbed through the gastrointestinal (GI) tract.Examples The oral BA of IR gabapentin is ~60% at a dose of 900 mg/day. In the absorption of gabapentin from small intestine, an L-amino acid transport system is involved, which is saturable. This saturable transporter is reportedly responsible for less than dose-proportional increase in gabapentin exposure with increasing doses. The bioavailability ICH guidance provides recommendations to support the biopharmaceutics classification of drug substances and the BCS-based biowaiver of bioequivalence studies for drug products. Download scientific diagram | The structural formulae of GABA (A) and gabapentin (B). from publication: Gabapentin: A pharmacotherapeutic panacea | Gabapentin (GBP) is a second generation Gabapentin, a structural analog of gamma-aminobutyric acid (GABA), a BCS class III drug, has permeability issues that lead to bioavailability problems. To overcome these issues, gabapentin is chemically conjugated with phosphatidylcholine and loaded as nanostructured lipid carriers (NLCs), which can be targeted to Phospholipase A2. Abstract. The biopharmaceutical classification system (BCS) classifies compounds based on their solubility and permeability. Regulatory agencies and health organizations have utilized this classification system to allow dissolution to be used to establish bioequivalence for highly soluble and highly permeable compounds. Table 4, Biopharmaceutics Classification System of antiepileptic medications - Effectiveness and Safety of Antiepileptic Medications in Patients With Epilepsy Your browsing activity is empty. Activity recording is turned off. The Biopharmaceutics Classification System (BCS) classifies pharmaceutical compounds based on their aqueous solubility and intestinal permeability. gabapentin demonstrates dose-dependent Related Searches: online bcs classification, bcs class 2 drugs list, bcs classification of drugs and its significance pdf, online bcs classification, bcs classification with examples, bcs class 3 drugs list, bcs classification fda, application of bcs classification, bcs class 4 drugs examples, potent cytotoxic drugs comes under what bcs classification, who bcs classification list. The Biopharmaceutics Classification System (BCS) is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability. This classification system was devised by Amidon et al.[1] This concept underlying the BCS published finally led to introducing the possibility of waiving in vivo Gabapentin was encapsulated in PLGA nanoparticles as it belongs to BCS class III, with low solubility and low permeability thus make it a suitable choice for delivering via surface modified nanoparticles so as to increase bioavailability. Chitosan coated PLGA nanoparticle loaded with gabapentin were prepared by nanoprecipitation method. Case I: anti-epileptic drugs considers BCS classification that can have a significant effect on absorption. BCS class II (carbamazepine, lamotrigine and phenytoin) and BCS class III BCS-based biowaivers are applicable to drug products where the drug substance(s) exhibit high solubility and, either high permeability (BCS Class I) or low permeability (BCS Class III). A biowaiver is applicable when the drug substance(s) in test and reference products are identical. The Biopharmaceutical Classification System (BCS) has been a prognostic tool for assessing the potential effects of formulation on the human drug oral bioavailability. When used in conjunction with in vitro dissolution tests, the BCS can support the prediction of in vivo product performance and the development of mechanistic models that support formulation assessments through the generation of Gabapentin enacarbil is classified as a BCS Class 2 compound (low solubility, high permeability). The applicant provided adequate information regarding structure elucidation and confirmation, According to Biopharmaceutics Classification System (BCS), gabapentin enacarbil is classified as a BCS Class 2 compound which have a low solubility and a high permeability. Limited drug absorption due to poor solubility of BCS Class 2 drug results in poor bioavailability, ultimately hampering its therapeutic effectiveness.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |