Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
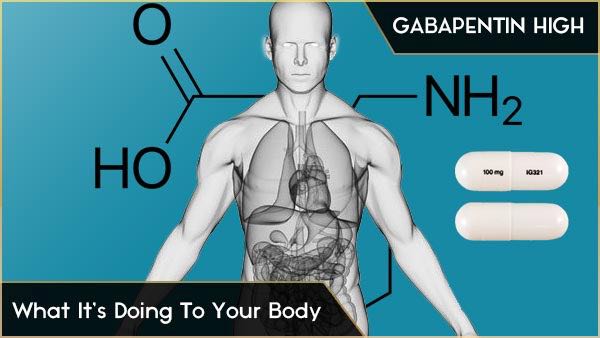
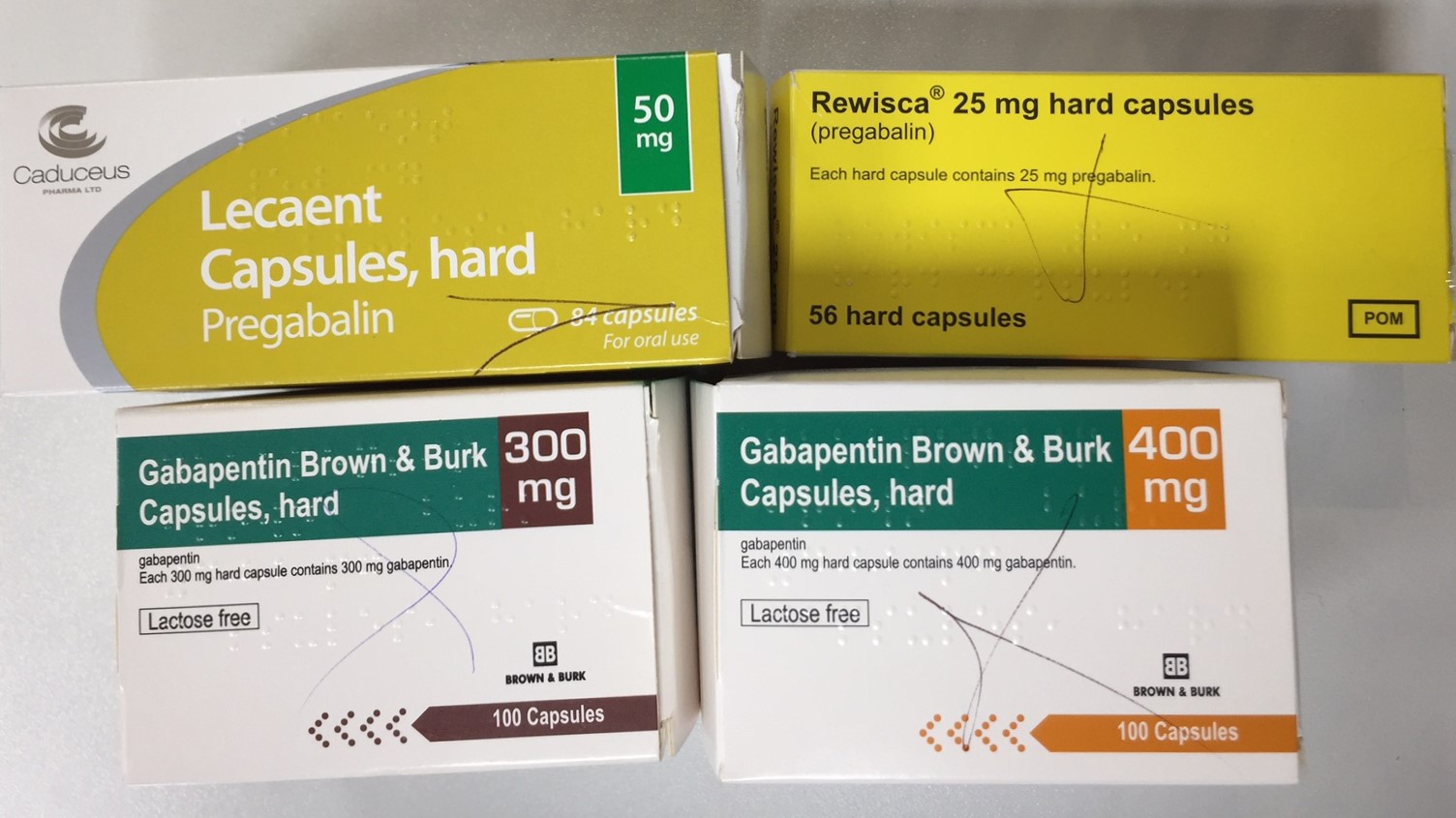
FAQ’s Gabapentin Reporting for Animal Shelters and Rescues ; S.L. 2023-65 amends GS 90-106 . Requires a practitioner to electronically prescribe all controlled substances in GS 90-93(a)(1)a (not more than 200 milligrams of codeine or any of its salts per 100 milliliters or per 100 grams) effective January 1, 2024. Resources The petition also pointed to studies suggesting that both gabapentin and pregabalin, which is marketed as Lyrica and is already classified as a Schedule 5 controlled substance, pose an addiction risk for patients with current or past substance abuse. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food Gabapentin is not a controlled substance on a federal level but is controlled in some states, which limits the number of prescription refills and how it is reported. Gabapentin can be dangerous when used in combination with other substances, particularly opioids. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73(b) adds it to the medications recorded in NC CSRS because it may cause a level of sedation in patients that puts them at increased risk of overdose when taken with opioids. The NC Department of Health and Human Services (NCDHHS) has As of September 2022, gabapentin was classified as a controlled substance in Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia. 6,7 Adding gabapentin to the list of controlled substances has required providers to have a Drug Enforcement Administrationregistration number to prescribe it, adding another layer of Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Schedule-V controlled substance and mandated reporting to PDMP. The State of Kentucky is, and to date, remains, the only state to have reclassified gabapentin as a Schedule-V controlled substance. 21 Effective July 1, 2017, the prescribing of gabapentin is limited to authorized practitioners, defined as practitioners registered with the US DEA. 21 Thus, mid-level practitioners, specifically Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Gabapentin is currently not a controlled substance under federal law. Gabapentin is often prescribed to treat conditions such as neuropathic pain, restless leg syndrome, and epilepsy. Gabapentin was first brought to the market as brand Neurontin in 1993 and since then has become widely used in the treatment of pain. For controlled substance licensure, the rule changes require a designated prescriber to have a controlled substance license for a health facility if substances are stored there without an on-site pharmacy or an automated device stocked by a pharmacy, provide an exception to licensure for an emergency kit that contains controlled substances Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly Gabapentin is not currently controlled under the Controlled Substances Act of 1970. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |