Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
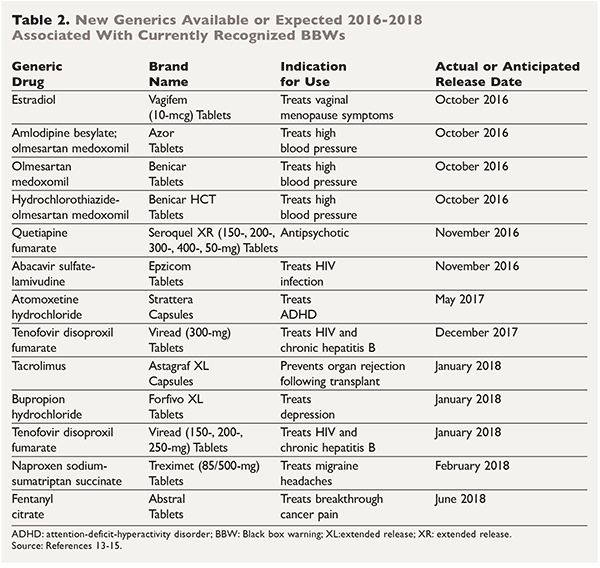
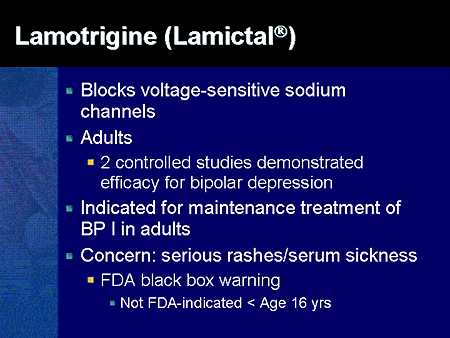
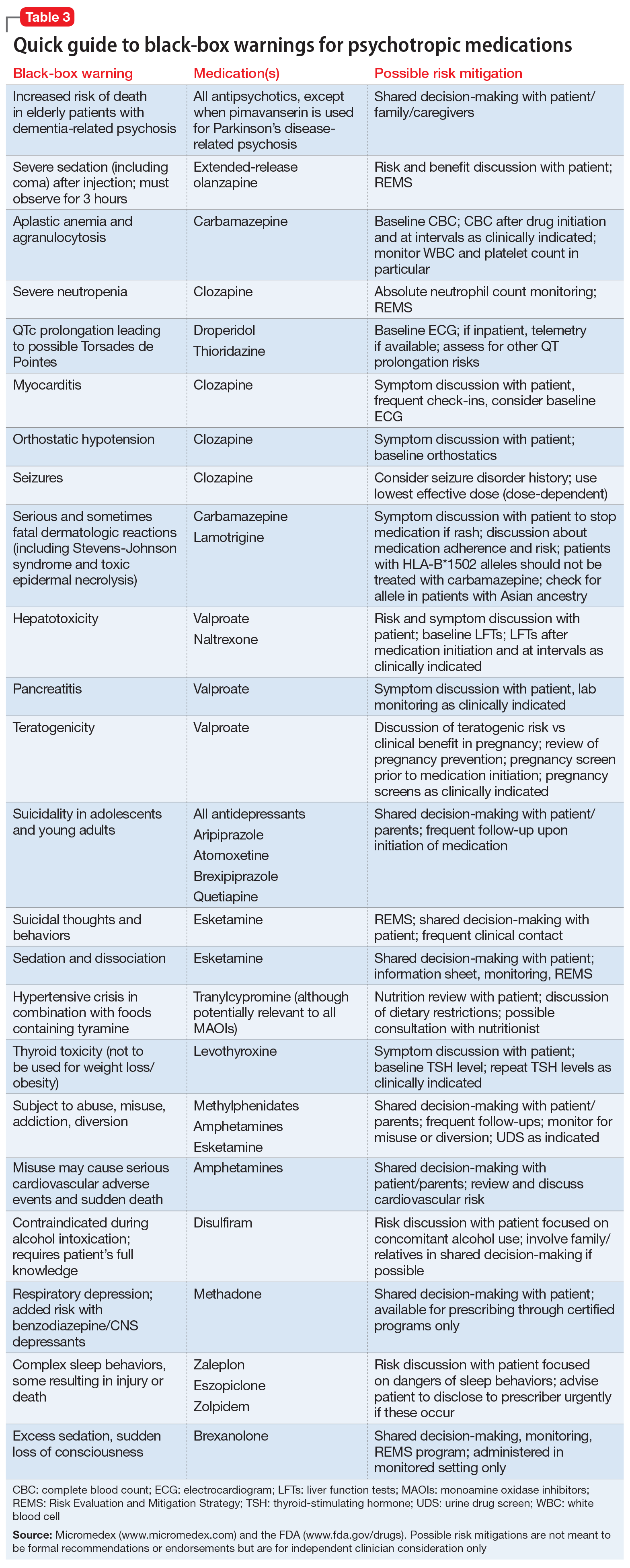
Consumers and health professionals are advised that Boxed Warnings are being added to the Product Information (PI) and Consumer Medicine Information (CMI) for medicines containing pregabalin and gabapentin. The enhanced warnings advise that pregabalin poses a risk of misuse, while both pregabalin and gabapentin pose risks of abuse and dependence. A black box warning – often referred to as simply a “boxed warning” – is the strongest warning issued by the FDA in the United States on drugs that carry specific health risks – serious or life-threatening adverse effects. The U.S. Food and Drug Administration (FDA) is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR The FDA recently released a warning for the medications, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). The FDA warned that serious breathing difficulties may occur in patients using these medications who have respiratory risk factors. FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in patients who use gabapentanoids with opioid pain medicines or other drugs that depress the The Food and Drug Administration is requiring that a new warning be added to the labeling of gabapentinoids concerning the risk of respiratory depression, especially when the drug is combined with other central nervous system (CNS) depressants or the patient has respiratory risk factors. The new warnings may lead people to file drug lawsuits over breathing-related injuries blamed on gabapentin’s and pregabalin’s respiratory risks. Poison control centers have reported increased calls about the gabapentinoids. Therapeutic and Goods Administration (TGA), Health authority of Australia released a box warning for medicines containing pregabalin and gabapentin for the risks of drug misuse, abuse, and dependence based on the continuous emerging safety data pertaining to these safety concerns. The agency is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Among those factors are use of opioid pain medicines and other drugs that depress the central nervous system (CNS), as well as conditions such as The FDA issued a warning in December 2019 that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors.¹ The risk factors include the use of opioid analgesics and other drugs that depress the central nervous system (CNS) and These so-called “black box warnings,” given the border often found around them, are required by the U.S. Food and Drug Administration (FDA) for certain medications that carry serious safety risks. In late December 2019, the U.S. Food and Drug Administration (FDA) announced that it will require new warning labels for gabapentinoids. These labels will address the risk of serious respiratory distress leading to death in patients who combine the treatment with an opioid. Abstract. The United States Food and Drug Administration issued a Black Box warning in October 2004 after placebo-controlled trials of antidepressant medications found an increased risk of suicidal thoughts and behaviors among children and adolescents taking antidepressant medications relative to placebo. ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors A black box warning is the FDA’s most stringent warning for drugs and medical devices on the market. Black box warnings, or boxed warnings, alert the public and health care providers to serious side effects, such as injury or death. The FDA requires drug companies to add a warning label to medications that have a black box warning. On July 10, 2008, an FDA scientific advisory committee voted “yes” that there was a significant positive association between AEDs and suicidality but voted against placing a black box warning on AEDs for suicidality. On December 16, 2008 FDA issued a label warning for heightened risk of suicidal thoughts and behavior for users of AEDs. Summary Background. Gabapentin, opioids, and/or benzodiazepines are commonly prescribed for a variety of pain and psychiatric conditions. Despite the high likelihood of co-prescription of these medications, little is known about co-utilization of gabapentin (GABA), opioids (OP), and benzodiazepines (BZD) and associated public health outcomes. On December 19, 2019 FDA is warning that serious breathing difficulties may occur in patients using gabapentin (brand names Neurontin, Gralise, Horizant) or pregabalin (brand names Lyrica, Although a black-box warning was not approved, the FDA collected data on the use of 11 anti-epileptic medications, including gabapentin, between 2005 and 2007 to determine whether there was indeed an increased risk of suicidal ideation or behavior. Gabapentin is the sixth most prescribed drug in the USA. So the FDA wants everybody to take notice. The new gabapentin warnings are strong and direct. They result from incidences between 2012 and 2017, where the FDA said it received over 50 reports of "great concern". The reports described respiratory problems associated with using gabapentin
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |