Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
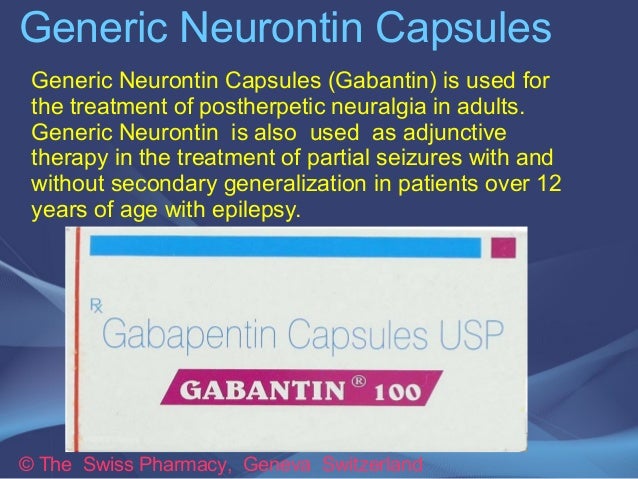
Public Assessment Report Scientific discussion Gabapentin Brown, capsule, hard, (gabapentin) SE/H/1003/01-03/DC This module reflects the scientific discussion for the approval of Gabapentin Brown. The procedure was finalised at 2011-10-11. For information on changes after this date please refer to the module ‘Update’. Taking into account the PRAC Assessment Report on the PSUR(s) for gabapentin, the scientific conclusions are as follows: In view of the available data, including post-marketing reports and the literature review, there is sufficient evidence for a causal relation between in-utero exposure to gabapentin and the occurrence of Based on the review of the quality, safety and efficacy data, the member states have granted a marketing authorisation for Gabapentine Sandoz capsules 100, 300 and 400 mg, from Sandoz B.V. The date of authorisation was on 7 August 2007 in the Netherlands. We would like to show you a description here but the site won’t allow us. We would like to show you a description here but the site won’t allow us. Primary objective To assess the bioequivalence of Gabapentin Capsule USP 300 mg of Lincoln Pharmaceuticals Ltd., India with NeurontinR (Gabapentin 300 mg) of Pfizer Ltd. UK in 24 normal, healthy, Lyrica is a medicine that contains the active substance pregabalin. It is available as capsules (white: 25, 50 and 150 mg; white and orange: 75, 225 and 300 mg; orange: 100 mg; light orange: 200 mg) and as an oral solution (20 mg/ml). Public Assessment Report Scientific discussion Gabapentine Mylan 600 mg and 800 mg, tablets (gabapentin) NL/H/5084/001-002/DC Date: 25 November 2019 This module reflects the scientific discussion for the approval of Gabapentine Mylan. The procedure was finalised on 7 June 2011 with Germany as RMS (DE/H/2828/001-002/DC). We would like to show you a description here but the site won’t allow us. additional tests: uniformity of mass of capsule content, uniformity of mass of total capsule, average weight of capsule shell, uniformity of dosage unit, disintegration test, dissolution test & microbiological tests. All limits are acceptable. Analytical methods were adequately described and validated. PUBLIC ASSESSMENT REPORT of the Medicines Evaluation Board in the Netherlands Gabapentine Sandoz capsules hard, 100, 300 and 400 mg Sandoz B.V., the Netherlands Gabapentin This assessment report is published by the MEB pursuant Article 21 (3) and (4) of Directive 2001/83/EC. The report This is a summary of the public assessment report (PAR) for Gabapentine Glenmark. It explains how this medicine was assessed and its authorisation recommended as well as its conditions of use. Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. A comprehensive description of the indications and posology is given in the SmPC. Gabapentin Orion hard capsules 300 mg and 400 mg contains as active substance 300 mg and 400 mg of gabapentin, respectively. Gabapentin Orion hard capsules 300 mg have a yellow cap and yellow body. They are imprinted with ‘D’ and ‘03’, and the length of the capsules is 20 mm. gabapentin 400 mg capsules with Neurontin 400 mg hard capsules under fasting conditions. Blood samples for concentration analysis were collected pre-dose and up to 48 hours post-dose. This is a summary of the Public Assessment Report (PAR) for Gabapentin Strides 100, 300 and 400 mg capsules, hard. It explains how these products were assessed and their authorisation Summary Public Assessment Report Gabapentin Orion gabapentin, hard capsules 300 mg and 400 mg This is a summary of the public assessment report (PAR) for Gabapentin Orion. It explains how Gabapentin Orion was assessed and its authorisation recommended as well as its conditions of use. We would like to show you a description here but the site won’t allow us. Public Assessment Report Scientific discussion Gabapentine 50 mg/ml Focus, oral solution (loperamide hydrochloride) NL License RVG: 125650 Date: 21 December 2020 This module reflects the scientific discussion for the approval of Gabapentine 50 mg/ml Focus, oral solution. Based on Scientific assessment & applied data for the submitted medicinal product: The applicant did not submit clinical data or scientific evidence supporting the use of topical gabapentin especially the mechanism of action of gabapentin depend on High affinity gabapentin binding sites have been
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |