Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
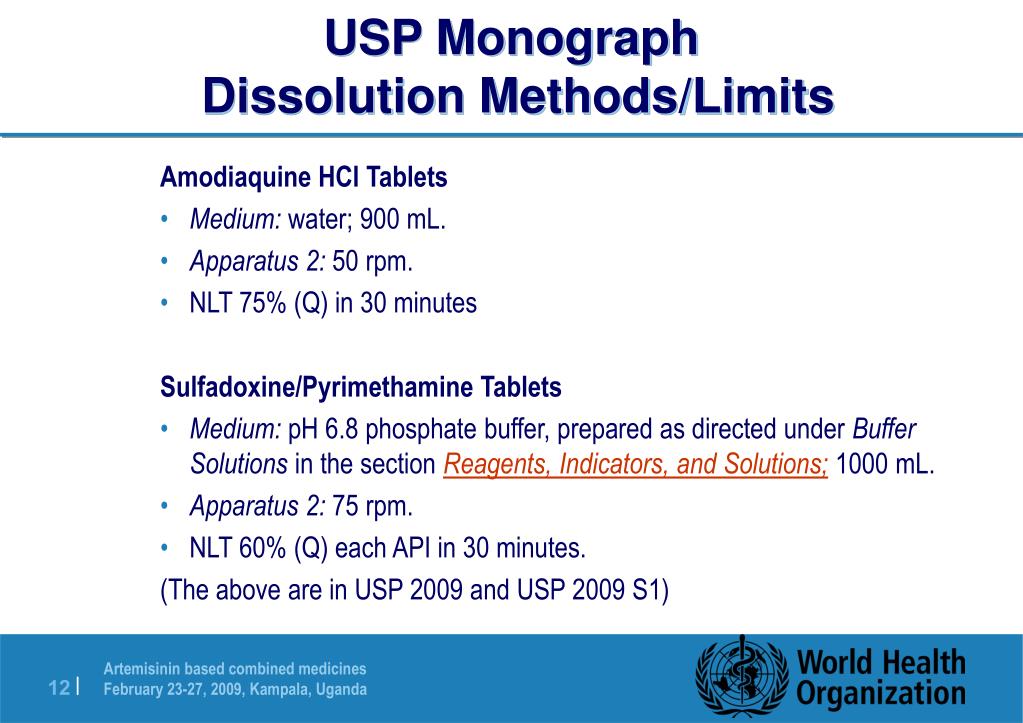
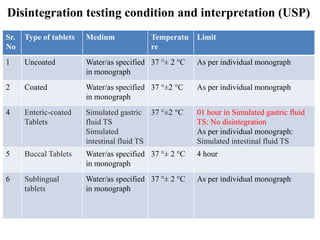
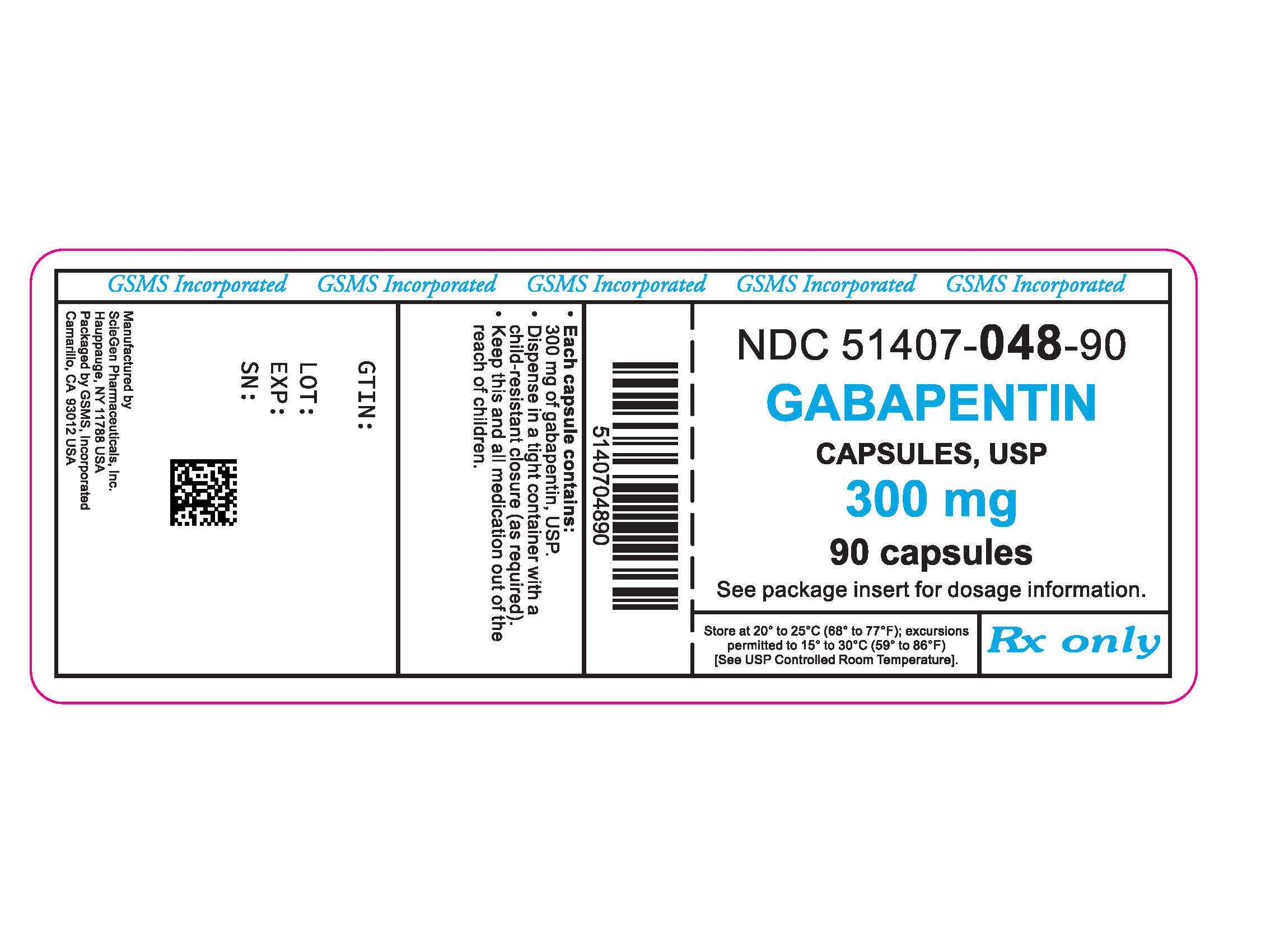
PRODUCT MONOGRAPH Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg ANTIEPILEPTIC AGENT Mylan Pharmaceuticals ULC 85 Advance Road, Etobicoke, ON M8Z 2S6 Submission Control No.: 202563 Date of Revision: February 14, 2017 Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (2024). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD Gabapentin Product Monograph Page 1 of 36 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrGabapentin Capsules USP Capsules 100 mg, 300 mg, and 400 mg, oral PRODUCT MONOGRAPH Prpms-GABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg Gabapentin Tablets, USP 600 mg and 800 mg Antiepileptic Agent PHARMASCIENCE INC. 6111 Royalmount Ave., Suite 100 Montréal, Québec H4P 2T4 www.pharmascience.com Date of Revision: May 17, 2018 Submission Control No: 215700 USP 35 Official Monographs / Gabapentin3297 Procedure—Separately inject equal volumes (about 20 µL) of. the Standard solution and the Test solution into the chromato-Gabapentin Capsules graph, record the chromatograms, and measure the responses for the major peaks. [NOTE—Disregard all the peaks having rela-» Gabapentin Capsules contain System suitability solution—Dissolve a suitable quantity of USP Gabapentin RS in Diluent, and add an appropriate volume of Impurities solution to obtain a solution containing about 14.0 mg per mL, 0.014 mg per mL, and 0.0084 mg per mL of USP. Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. APO-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). Gabapentin Capsules, USP DESCRIPTION Gabapentin Capsules, USP are supplied as imprinted hard gelatin capsules containing 100 mg, 300 mg and 400 mg of gabapentin, USP. The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide. The 300 mg capsule contains FD&C Red 40, D&C Yellow 10 The Gabapentin Tablets Revision Bulletin supersedes the monograph in USP 32–NF 27 until it is printed in the USP 33–NF 28 First Supplement which will be released 1 February 2010 and becomes official 1 August 2010. Should you have any questions, please contact Margareth Marques, Ph.D. (301-816-8106 or mrm@usp.org). Gabapentin enacarbil is used for symptomatic treatment of moderate-to-severe primary restless legs syndrome (Ekbom syndrome) in adults. Not recommended in patients who are required to sleep during the daytime and remain awake at night. Priva-GABAPENTIN Product Monograph Page 4 of 31 WARNINGS AND PRECAUTIONS General Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) is not considered effective in the treatment of absence seizures and should therefore be used with caution in patients who JAMP Gabapentin Capsules (Product Monograph) Page 1 of 28 PRODUCT MONOGRAPH PrJAMP Gabapentin Capsules Gabapentin Capsules Capsules, 100 mg, 300 mg, and 400 mg, Oral USP The Product Monograph is a scientific document that describes the properties, claims, indications and conditions of use of the product and contains any other information that may be required for optimal, safe and effective use. Buy [Gabapentin (250 mg)] - CAS [60142-96-3] from USP. * Certain Material Origins (i.e. Animal, Plant, Fish) may require special country importation requirements.USP recommends you contact your country competent authorities to determine if any certifications, permits or licenses may be required prior to ordering.Material Origins are found within the Product under Origin Information. PRODUCT MONOGRAPH PrTEVA-GABAPENTIN Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Teva Standard Teva Canada Limited 30 Novopharm Court, Toronto, Ontario Date of Revision: December 6, 2017 Canada M1B 2K9 www.tevacanada.com Submission Control No: 211039 Assay preparation— Remove and weigh the contents of not fewer than 20 Capsules. Transfer an accurately weighed portion of the powder, equivalent to about 100 mg of gabapentin, to a suitable volumetric flask, and dissolve the contents in Diluent with sonication, if necessary, for about 60 seconds. 2S (USP32) USP Gabapentin RS and USP Gabapentin Related Compound A RS Medium: 0.06N hydrochloric acid (prepared by adding 51mL in Diluent to obtain a solution having a known concentration of of hydrochloric acid to 10 L of water); 900mL. about 0.04mg of each per mL. Apparatus 2:50 rpm. Test solution—Weigh and finely powder not fewer than 20 Tab- Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |