Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
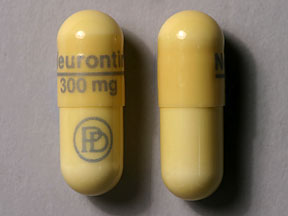
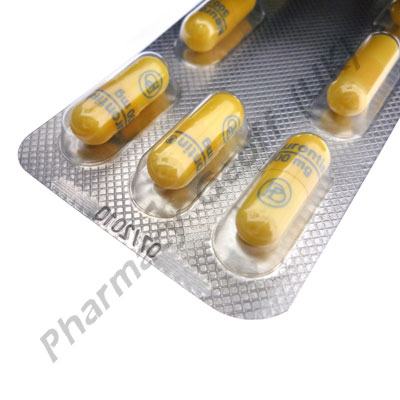
Gabapentin Capsules, USP are available containing 100 mg, 300 mg or 400 mg of gabapentin, USP, supplied as follows: 100 mg capsules: Size '3' Hard gelatin capsules with white opaque cap and white opaque body, imprinted "100 mg" in blue ink on cap and "236" in blue ink on body, filled with white to off-white powder. Gabapentin Capsules, USP 300 mg are available for oral administration as hard gelatin capsules with a white opaque body and a yellow opaque cap. “APO 113” is imprinted on each capsule in black ink; supplied in: Gabapentin Capsules USP are supplied as follows: 100 mg capsules: White hard gelatin capsules imprinted “216” on body with blue ink, available in: Cartons of 100 capsules (10 capsules each blister pack x 10), NDC 0904-6665-61 The active ingredient in gabapentin capsules, USP is gabapentin USP, which has the chemical name 1-(Amino methyl)-cyclohexane acetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Gabapentin USP is a white to off-white powder. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. PRECAUTIONS, Special Populations). Gabapentin Capsules, USP are supplied as imprinted hard gelatin capsules containing 100 mg, 300 mg and 400 mg of gabapentin, USP. The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide. The 300 mg capsule contains FD&C Red 40, D&C Yellow 10 and titanium dioxide. Gabapentin Capsules USP and Gabapentin Tablets USP dose is reduced, discontinued or substituted with an alternate anticonvulsant or an alternate anticonvulsant is added to Gabapentin Capsules USP and Gabapentin Tablets USP therapy, this should be done gradually over a minimum of 1 week (a longer GABAPENTIN CAPSULES, USP, 100 mg, 300 mg and 400 mg GABAPENTIN TABLETS, USP, 100 mg, 300 mg, 400 mg, 600 mg and 800 mg Manufactured by Manufactured for Apotex Inc. Apotex Corp. Toronto, Ontario Weston, Florida Canada M9L 1T9 33326 Revised: June 2015 Rev. 2 Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. USP REFERENCE STANDARDS FOR PURCHASE USP Gabapentin RS maintenance dose of gabapentin capsules is 300 mg to 600 mg three times a day. D. sages up to 2400 mg/day have been well tolerated in long-term clinical studies. Do. es of 3600 mg/day have also been administered to a small n. minister gabapentin capsules three times a day using 300 m. Gabapentin Capsules, USP is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. Gabapentin Capsules, USP is also indicated as adjunctive therapy in the treatment of partial seizures in pediatric patients age 3 to 12 years. Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Gabapentin can cause life-threatening breathing problems, especially if you already have a breathing disorder or if you use other medicines that can make you drowsy or slow your breathing. Read the Medication Guide before you start taking gabapentin and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. What is the most important information I should know about gabapentin? Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin USP. The inactive ingredients for the capsules are calcium carbonate, calcium sulfate dihydrate, glyceryl behenate, and pregelatinized starch. The active ingredient in Gabapentin Capsules USP is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Gabapentin is a white to off-white crystalline solid with a pK a1 of 3.7 and a pK a2 Store gabapentin capsules at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep gabapentin capsules and all medicines out of the reach of children. 2S (USP32) USP Gabapentin RS and USP Gabapentin Related Compound A RS Medium: 0.06N hydrochloric acid (prepared by adding 51mL in Diluent to obtain a solution having a known concentration of of hydrochloric acid to 10 L of water); 900mL. about 0.04mg of each per mL. Apparatus 2:50 rpm. Test solution—Weigh and finely powder not fewer than 20 Tab- Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. USP Gabapentin Related Compound A RS . A: Infrared Absorption 197K. Test specimen Empty the contents of not fewer than 10 Capsules, and grind to a fine powder. Use an amount of the powder, equivalent to 2 mg of gabapentin, and 200 mg of potassium bromide. Gabapentin capsules, USP are a prescription medicine used to treat: Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |