Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 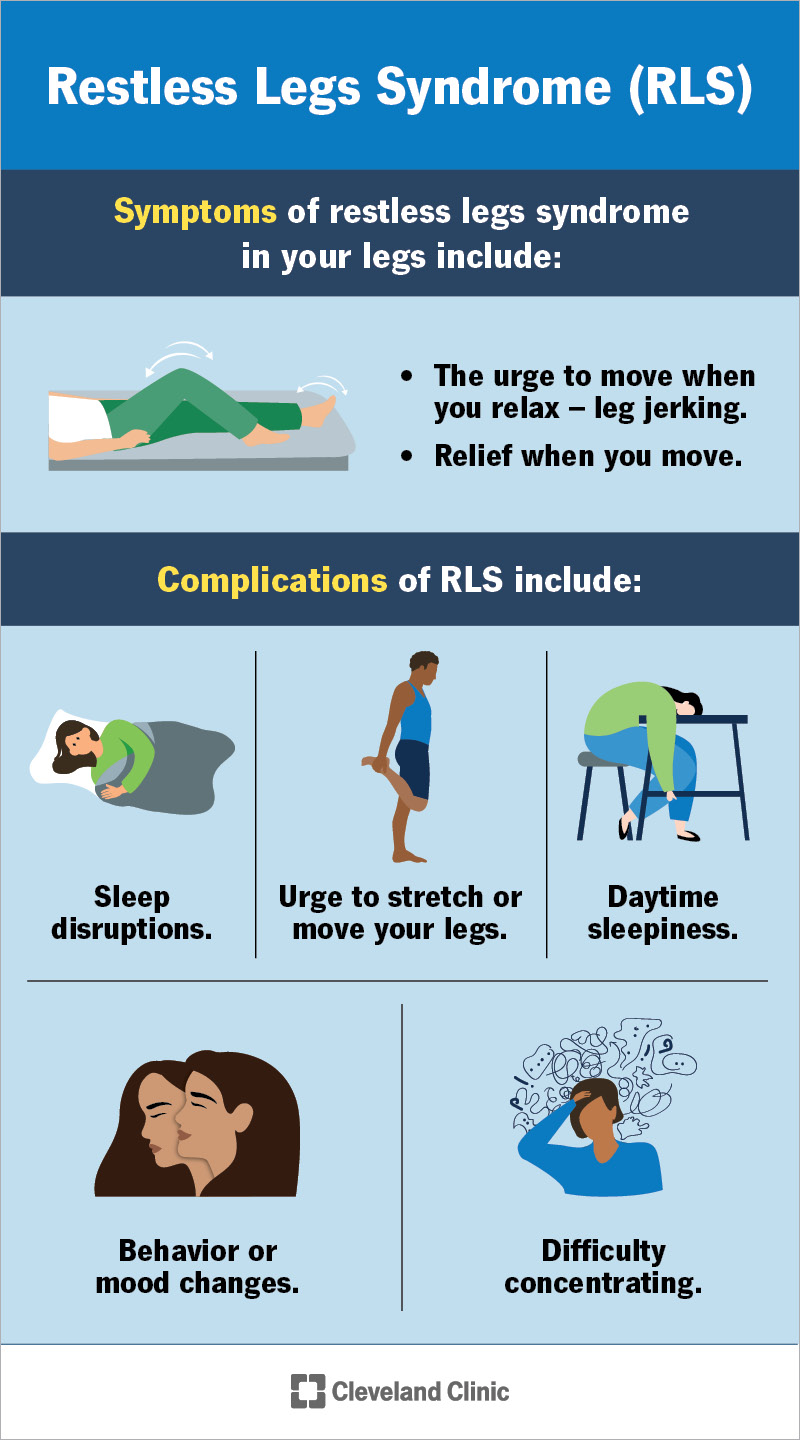 |
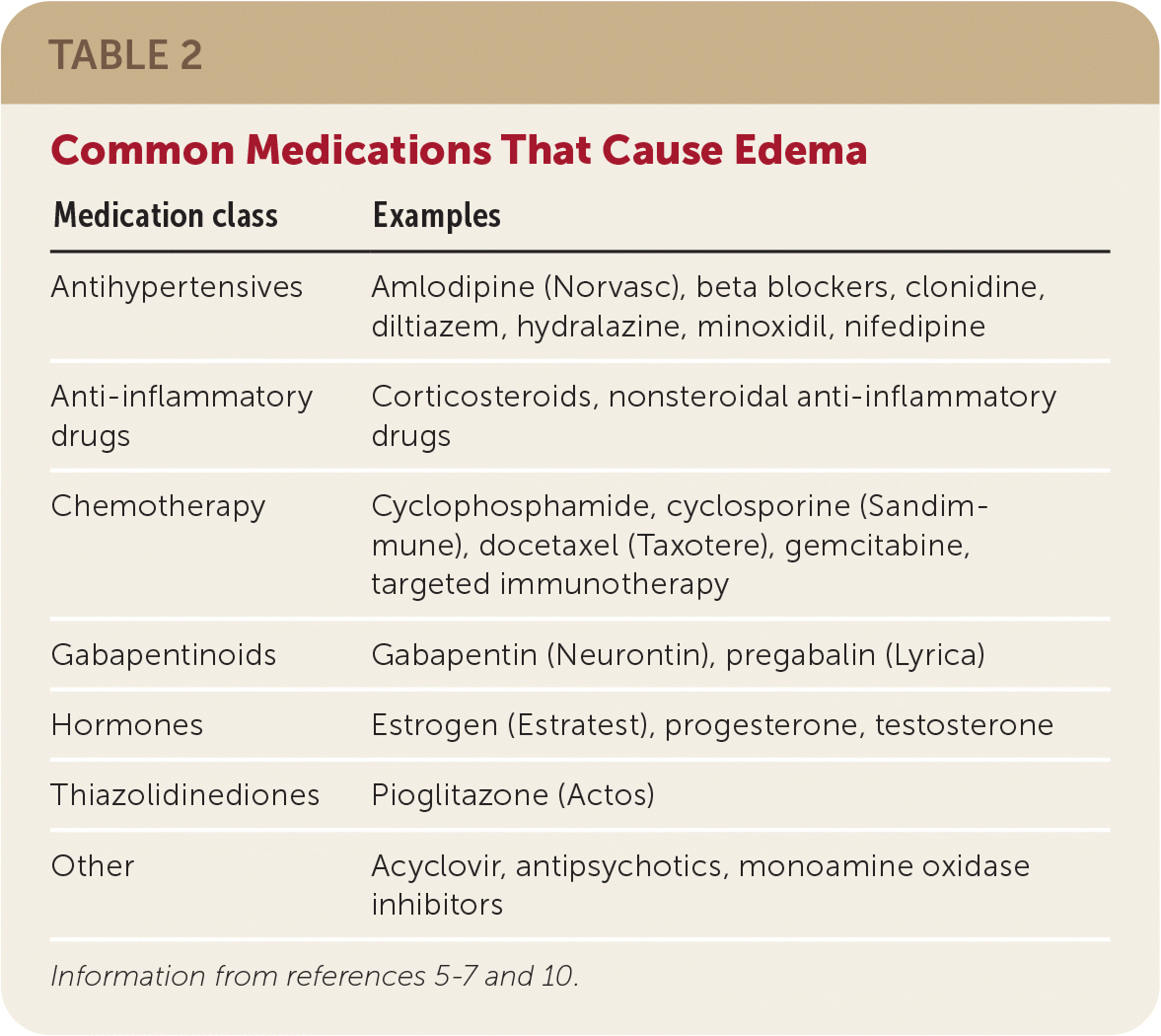
Currently, the US Food and Drug Administration (FDA) has only approved gabapentin enacarbil for RLS, but there are other antiseizure drugs that are also prescribed “off-label” for RLS. Off-label means that prescribing these drugs for RLS is not authorized by the FDA. These drugs include: 1-3. The FDA approved gabapentin enacarbil in 2011 as the first non-dopaminergic agent for the treatment of restless legs syndrome (RLS) symptoms. Although gabapentin enacarbil is a pro-drug of gabapentin, its pharmacokinetics differ. Gabapentin enacarbil is used to treat moderate-to-severe primary Restless Legs Syndrome (RLS). RLS is a neurologic disorder that makes the legs feel uncomfortable. This results in an irresistible feeling of wanting to move your legs to make them comfortable. Objective: To assess the effects of gabapentin on sensory and motor symptoms in patients with restless legs syndrome (RLS). Methods: Patients with RLS (22 idiopathic, 2 secondary to iron deficiency) were randomized and treated for 6 weeks with either gabapentin or placebo. In contrast, new evidence supporting three alpha-2-delta ligand calcium channel blockers — gabapentin enacarbil, gabapentin, and pregabalin — led the task force to support them as strong recommendations for RLS treatment. These medications are not associated with the augmentation of RLS symptoms observed with the dopaminergic agents. Restless legs syndrome (RLS) is a neurologic movement disorder that affects approximately 10 percent of adults. 1 – 3 About one third of those with RLS have symptoms of moderate to severe Objective: To assess the effects of gabapentin on sensory and motor symptoms in patients with restless legs syndrome (RLS). Methods: Patients with RLS (22 idiopathic, 2 secondary to iron deficiency) were randomized and treated for 6 weeks with either gabapentin or placebo. Serum ferritin is a marker for tissue iron-load status in the body, and low serum-ferritin levels (< 75 µg/L) serve as a rationale for starting iron-replacement therapy in a patient with restless leg syndrome, particularly when symptoms have worsened (i.e., at time of initial diagnosis, when symptoms progress, or in the setting of augmentation This article explains what gabapentin is, its approved and off-label uses, and how the drug works to treat restless legs syndrome and other medical conditions. It also describes the possible side effects and risks and lists other drugs and treatments that may help ease RLS symptoms. A. Gabapentin enacarbil (Horizant) has been approved by the FDA for the treatment of restless legs syndrome (RLS) and postherpetic neuralgia (the pain that can linger after a bout of shingles). It is different from plain gabapentin ( Neurontin or Gralise ). Restless legs syndrome (RLS) is a common disorder. The population prevalence is 1.5% to 2.7% in a subgroup of patients having more severe RLS with symptoms occurring 2 or more times a week and causing at least moderate distress. It is important for primary care physicians to be familiar with the disorder and its management. Much has changed in the management of RLS since our previous revised Gabapentin has been shown to improve RLS in a small number of clinical studies, but is limited by its short half-life and variable bioavailability. Gabapentin enacarbil is a novel prodrug of gabapentin designed to overcome these pharmacokinetic limitations. Restless leg syndrome is reported as a side effect among people who take Gabapentin (gabapentin), especially for people who are female, 60+ old, have been taking the drug for < 1 month also take Xyrem, and have Narcolepsy. Restless legs syndrome is a disorder that causes an overwhelming urge to move the legs, usually to alleviate unpleasant sensations. Gabapentin enacarbil, Restless legs syndrome (RLS e104 Tzonova D, Larrosa O, Calvo E, et al. Breakthrough symptoms during the daytime in patients with restless legs syndrome (Willis-Ekbom disease). Sleep Med 2012;13:151–155. e105 Godau J, Spinnler N, Wevers AK, Trenkwalder C, Berg D. Poor effect of guideline based treatment of restless legs syndrome in clinical practice. Nine patients with idiopathic restless legs syndrome (RLS) were treated with 300 mg of gabapentin as an initial dose and an up-titration until relief of symptoms for 4 weeks. Subjective symptoms improved significantly. Gabapentin typically takes about 1 to 2 hours to start working for restless legs, providing relief from discomfort and restlessness. Restless Legs Syndrome (RLS) is a condition that can significantly impact one’s quality of life. Restless legs syndrome (RLS) is a common neurological disorder of unknown etiology that is managed by therapy directed at relieving its symptoms. Treatment of patients with milder symptoms that occur intermittently may be treated with improves RLS symptoms. The most common AEs were nausea and sleepiness/fatigue. Rotigotine It is highly likely that the rotigotine patch improves RLS symptoms. The most common AEs were mild skin reactions at the application site and nausea. Gabapentin enacarbil It is highly likely that gabapentin enacarbil improves RLS symptoms. Despite ameliorating RLS symptoms, GBP’s pharmacokinetic limitations restrict its overall effectiveness. A novel specifically designed prodrug, gabapentin enacarbil (GE), has demonstrated successful RLS alleviation with a superior pharmacokinetic profile.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |