Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
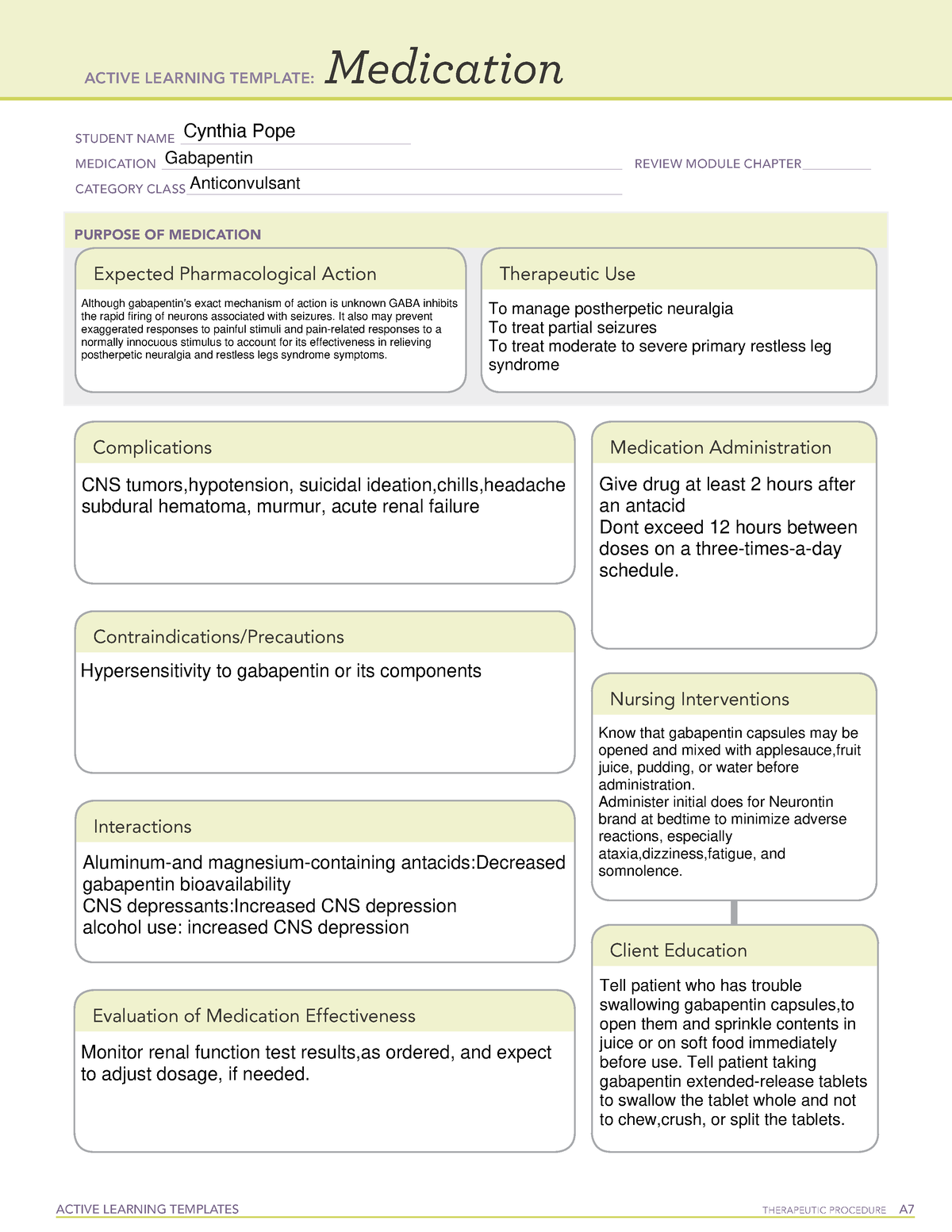 |  |
Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Last year the Advisory Council on the Misuse of Drugs recommended the drugs to be reclassified as class C drugs because of the risks of “illegal diversion and medicinal misuse”. Ministers have now accepted this recommendation and it will be implemented subject to consultation. Utah's Controlled Substance Database Program (CSD) is a resource that assists prescribing practitioners and pharmacists in providing efficient care for their patients and customers usage of controlled substances. We compared two interventions including (1) class V scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after Gabapentin is also sometimes used to relieve the pain of diabetic neuropathy (numbness or tingling due to nerve damage in people who have diabetes), and to treat and prevent hot flashes (sudden strong feelings of heat and sweating) in women who are being treated for breast cancer or who have experienced menopause (''change of life'', the end of monthly menstrual periods). , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Gabapentin: Class: Analgesics Chemistry: Structural analogue of GABA Routes of administration: Oral only. Absorption: Variable oral bioavailability; 60% in small doses, 40% in large doses, mainly because it is absorbed by saturable facilitated transport (L-amino acid transporter) Solubility DOPL: You are correct, pharmacies and pharmacists who fill and dispense gabapentin will be most impacted by the law change, as they must report the dispensing of gabapentin to the CSD. Providers who prescribe gabapentin will see no change to them other than the Provider will be able to view in the CSD if their patient has received an Rx for From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. The drugs – pregabalin and gabapentin – became illegal to possess without a prescription and illegal to supply or sell to others in the UK from April 2019. The move came after experts highlighted the potential for misuse, and rising numbers of fatalities linked to the drugs. 9 This bill adds gabapentin to the list of controlled substances. 10 Highlighted Provisions: 11 This bill: 12 < adds gabapentin to Schedule V of the list of controlled substances; and 13 < makes technical and conforming changes. 14 Money Appropriated in this Bill: 15 None 16 Other Special Clauses: 17 None 18 Utah Code Sections Affected: 19 AMENDS: While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73(b) adds it to the medications recorded in NC CSRS because it may cause a level of sedation in patients that puts them at increased risk of overdose when taken with opioids. The NC Department of Health and Human Services (NCDHHS) has The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Having originally been developed as an anti-seizure medication, gabapentinoids include gabapentin and pregabalin, which are now prescribed primarily for neuropathic pain, seizures and anxiety, but also for fibromyalgia, restless legs syndrome and complications of MS (Chan et al, 2023). Gabapentin is in a class of medications called anticonvulsants. What are the brand names of gabapentin? Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |