Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
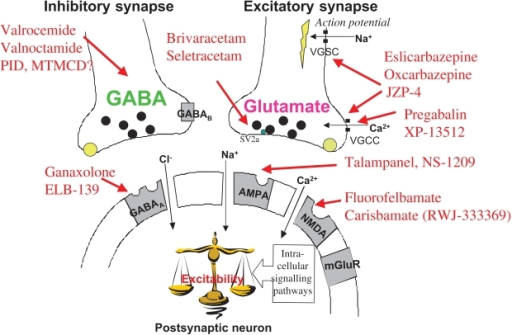
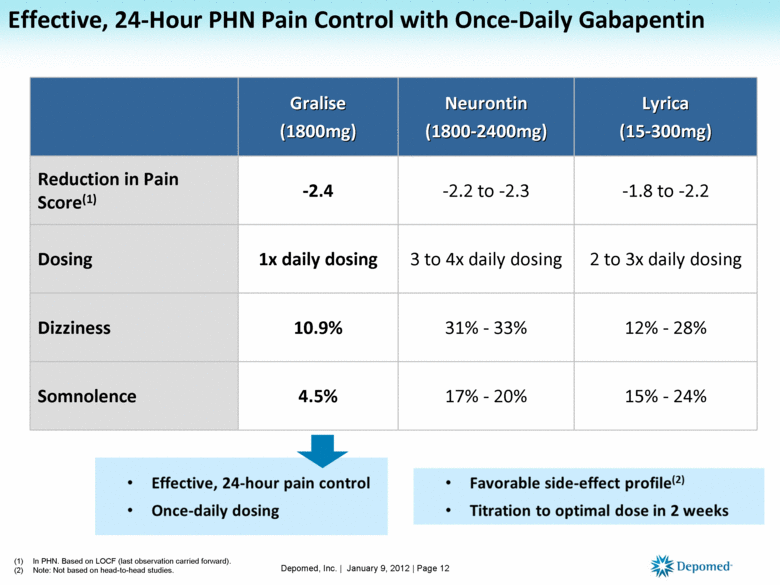
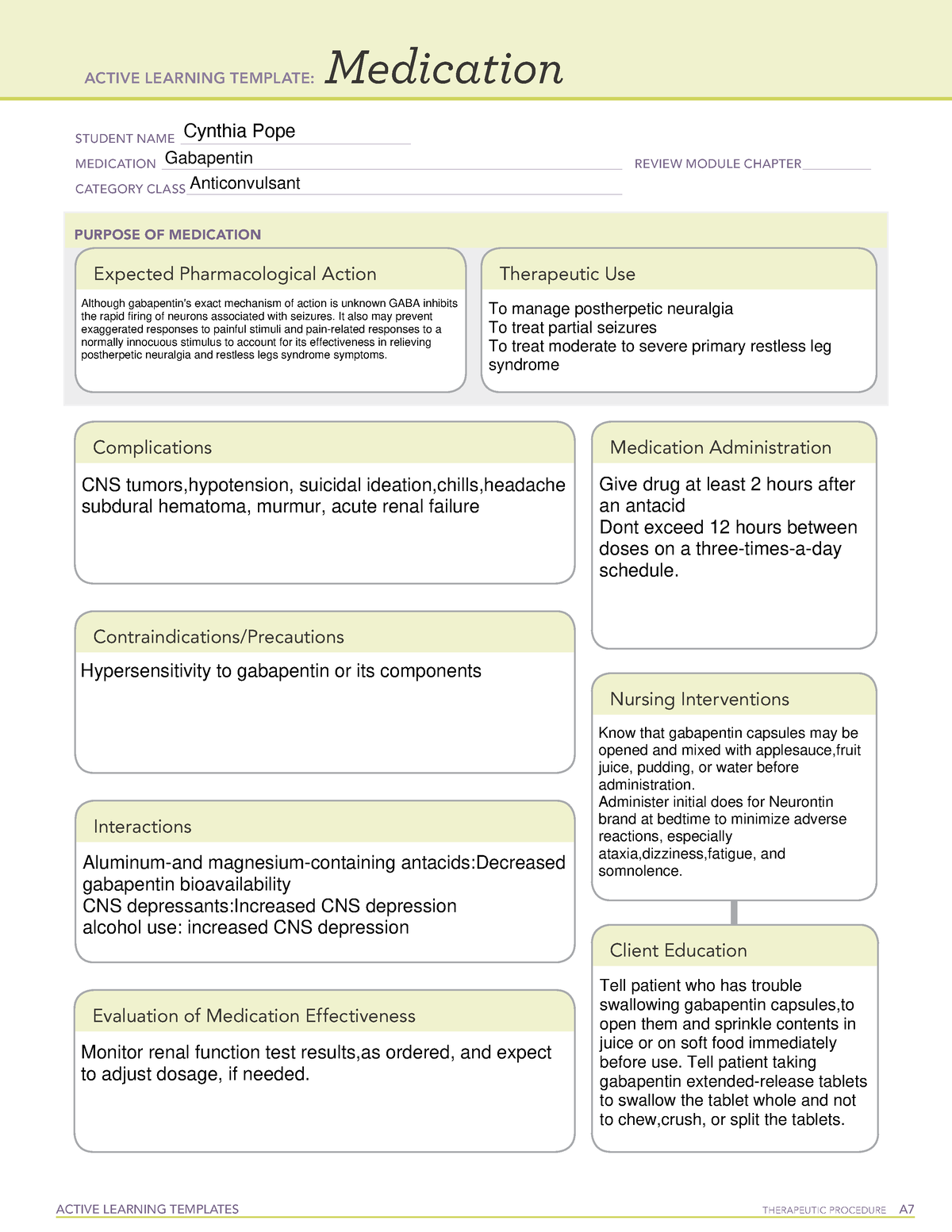
Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] . Gabapentin, initially developed for epilepsy, is now widely used for nerve pain and other off-label applications. Rising prescription rates have sparked discussions about whether it should be classified as a controlled substance due to concerns over misuse and dependency. Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. Update on Gabapentin in Ohio As a reminder, gabapentin is not considered a controlled substance in Ohio. The Board was made aware of incorrect communications made by a third-party vendor stating that Ohio had made gabapentin a controlled substance. While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Pharmacies submit prescription data to the PDMP system for all Schedules II, III and IV controlled substances, gabapentin, and naloxone dispensed from Oregon pharmacies and to Oregon residents from non-resident pharmacies. The protected health information is collected and stored securely. As of September 2022, gabapentin was classified as a controlled substance in Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia. 6,7 Adding gabapentin to the list of controlled substances has required providers to have a Drug Enforcement Administrationregistration number to prescribe it, adding another layer of Misuse and abuse: Gabapentin can be addictive and has the potential for misuse and abuse. Individuals taking this medication should be closely monitored for signs of misuse or abuse. Medication Interactions: Some medications can interact with gabapentin and cause dangerous side effects. It’s important to let your doctor know about any other Gabapentin is a prescription medication approved by the Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. It is currently marketed in capsule, tablet and oral solution formulations. Like gabapentin, it's taken for epilepsy and nerve pain. It can also be taken for anxiety. But there are differences between pregabalin and gabapentin. Pregabalin can be taken less often and in different doses to gabapentin. If you need to change to pregabalin, your doctor will explain how to swap safely from gabapentin. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how to treat them. Are they controlled drugs? In short, yes they are controlled drugs but no, they do not need to be locked in a CD cabinet, recorded in a CD register or given with a witness. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. on the United Kingdom recently reclassifying gabapentin as a Schedule III controlled drug in April 2019.27 The U.K. classifi-cation system is similar to the U.S. system in that there are 5 schedules, but it is slightly different in that the scheduling indicates the level of control compared to potential for abuse with the U.S. system.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |