Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
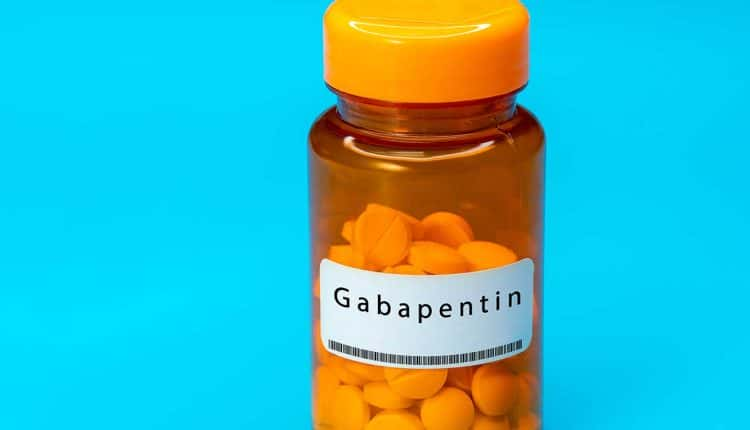
(1) A pharmacist may dispense directly a controlled substance listed in Schedule III, IV, or V which is a prescription drug, or any legend drug, only pursuant to either a written prescription signed by a prescribing individual practitioner or a facsimile of a written signed prescription transmitted directly by the prescribing practitioner, or the Each drug or substance has been assigned the Controlled Substances Code Number set forth opposite it. (a) An ephedrine combination product, pseudoephedrine, and phenylpropanolamine, as defined in § 5-64-1105, are designated Schedule V controlled substances in addition to the drugs and other substances listed in Schedule V of the List of Controlled Substances for the State of Arkansas promulgated by the Secretary of the Department of Health. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Each controlled substance or basic class thereof has been assigned an “Administration Controlled Substance Code Number” for purposes of identification. These numbers are for internal management and are used as a means to identify substances with complex and cumbersome chemical names. Next to the code number is the date the substance was (a) An ephedrine combination product, pseudoephedrine, and phenylpropanolamine, as defined in § 5-64-1105, are designated Schedule V controlled substances in addition to the drugs and other substances listed in Schedule V of the List of Controlled Substances for the State of Arkansas promulgated by the Secretary of the Department of Health. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky Controlled Substance List. The Arkansas Department of Health is responsible for maintaining and updating the controlled substance list for the State of Arkansas. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Arkansas State Board of Pharmacy Law Book Controlled Substances and Legend Drugs: May 2023 F . Controlled Substances and Legend Drugs . 20-64-501. Applicability . Nothing in this subchapter shall apply to the sale of chemicals or poisons for use for nonmedical purposes, or for uses Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and 36. Is Gabapentin a Controlled Substance in Tennessee and does it require a DEA to prescribe? Gabapentin is a Schedule V Controlled Substance in Tennessee and therefore should be treated just like any other Schedule V Controlled Substance. 37. I suspect my healthcare practitioner is engaged in TennCare fraud, waste, or abuse. What do I do? The agency licenses, permits, and oversees pharmacists and pharmacies, as well as the distribution system where there is the sale, delivery, or distribution of prescription drugs, medical gases, durable medical equipment, and legend devices. Schedule-V controlled substance and mandated reporting to PDMP. The State of Kentucky is, and to date, remains, the only state to have reclassified gabapentin as a Schedule-V controlled substance. 21 Effective July 1, 2017, the prescribing of gabapentin is limited to authorized practitioners, defined as practitioners registered with the US DEA. 21 Thus, mid-level practitioners, specifically (2) not more than 240 cc (8 ounces) of any such controlled substance containing opium, nor more than 120 cc (4 ounces) of any other such controlled substance nor more than 48 dosage units of any such controlled substance containing opium, nor more than 24 dosage units of any other such controlled substance may be dispensed at retail to the Each controlled substance or basic class thereof, has been assigned an “Administration Controlled Substance Code Number” for purposes of identification. These numbers are for internal management and are used as a means to (1) A Schedule I or Schedule II controlled substance that is methamphetamine or cocaine with an aggregate weight, including an adulterant or diluent, of: (A) Less than two grams (2g) upon conviction is guilty of a Class D felony; (2) not more than 240 cc (8 ounces) of any such controlled substance containing opium, nor more than 120 cc (4 ounces) of any other such controlled substance nor more than 48 dosage units of any such controlled substance containing opium, nor more than 24 dosage units of any other such controlled substance may be dispensed at retail to the
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |