Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
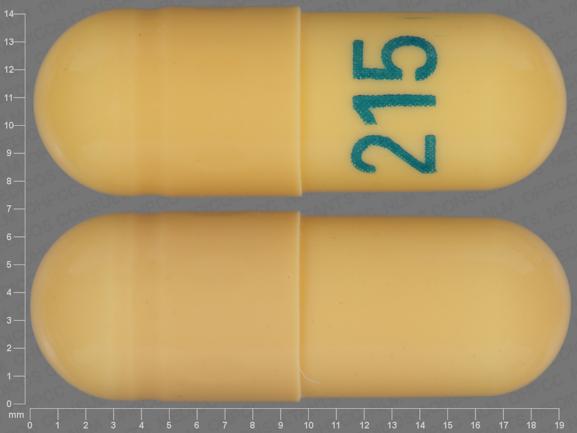
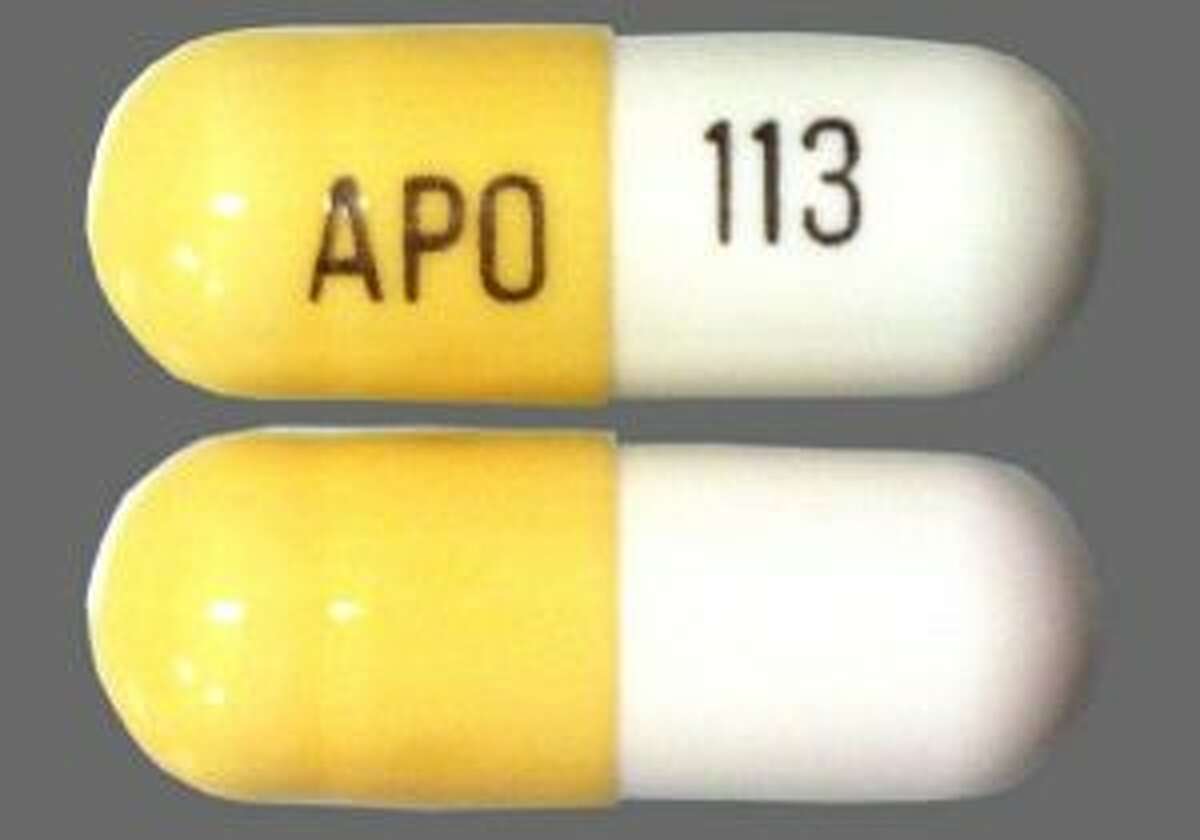
All legislative documents related to controlled substances are contained in the Controlled Drugs and Substances Act, the Narcotic Control Regulations, Parts G and J of the Food and Drug Regulations , and the Benzodiazepines and Other Targeted Substances Regulations. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. In general, medications classified as Schedule V (Schedule 5) controlled substances are considered to have the lowest potential for abuse compared to other OTTAWA – Health Canada is advising Canadians about the increased risk of opioid overdose and serious side effects when taking gabapentin (e.g., Neurontin) or pregabalin (e.g., Lyrica) with an opioid. Gabapentin is authorized to treat epilepsy and pregabalin is authorized to treat nerve pain. The illegal production and sale of controlled substances is linked with serious harm to public health and public safety. The Controlled Drugs and Substances Act and its regulations apply to controlled substances and precursor chemicals (chemicals used to make controlled substances). This federal law applies to the following activities with Gabapentin Abuse. Gabapentin has not been labeled as a controlled substance in Canada, but it is increasingly being used as an intoxicating agent, and gabapentin abuse is on the rise. Some people use it to increase the effects of opioids, and it’s also used as a cutting agent for heroin. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. To maintain Canadians’ access to controlled substances for medical treatments (e.g., treatment of substance use disorders and chronic pain), while they adhere to social distancing guidance from public health officials or if they need to self-isolate, Health Canada has issued the attached exemptions for prescriptions of controlled substances unde Ktm303 adalah sebuah agen situs yang menyediakan slot online gacor terpercaya yang hadir mejadi solusi yang tepat bagi para pecinta slot untuk pengalaman bermain ekslusif dan peluang menang maxwin hari ini, Ktm303 menjadi pilihan utama bagi para pecinta slot karena memiliki koleksi permainan yang memiliki kualitas tertinggi, siste, permainan modern dan inovatif serta hanyan dengan modal Gabapentin is a commonly prescribed medication, with about 3.9 mil- lion prescriptions filled in Canada in 2015. 1 Gabapentin is approved by Health Canada as adjunctive therapy in the management of epilepsy. 1 work with international organizations and other countries to meet Canada's obligations regarding controlled substances The regulations apply to a broad range of parties, including: manufacturers, distributors, importers and exporters who must get a licence to produce, sell, import or export controlled substances and precursors Any loss or theft of a narcotic, controlled drug, or targeted substance, including dispensed forgeries, must be reported to Health Canada within 10 days of discovery. All records must be kept in the pharmacy for a period of two years from the date that each record is made. This quick reference guide is not exhaustive. In 2018, the U.K. reclassified the drug as a controlled substance along the same lines as opioids. Some states in the U.S. have mandated the reporting of gabapentin prescriptions. As of September 2022, gabapentin was classified as a controlled substance in Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia. 6,7 Adding gabapentin to the list of controlled substances has required providers to have a Drug Enforcement Administrationregistration number to prescribe it, adding another layer of , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. initiatives which the publicly funded drug programs, colleges of physicians and surgeons, and colleges of pharmacy are implementing across Canada to address the misuse, abuse, and diversion of prescription narcotics (opioids), benzodiazepines, stimulants, and gabapentin. This information may assist drug policy Gabapentin is frequently combined with other substances for the purpose of potentiating the effects of the drugs or achieving a “high.” Studies have identified various substances that are commonly abused in combination with gabapentin, including alcohol, opioids, benzodiazepines, antidepressants, and other CNS depressants 13,14,15. Health Canada's review concluded that there is evidence supporting a risk of serious breathing problems when gabapentin is used. Health Canada recommended updates to the product information for gabapentin to warn about this risk. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Section C.01.020.1 of the Food and Drug Regulations and section 62 of the Medical Devices Regulations, require hospitals to report to Health Canada all serious adverse drug reactions (ADRs) and medical device incidents (MDIs) within 30 days of being documented within the hospital.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |