Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
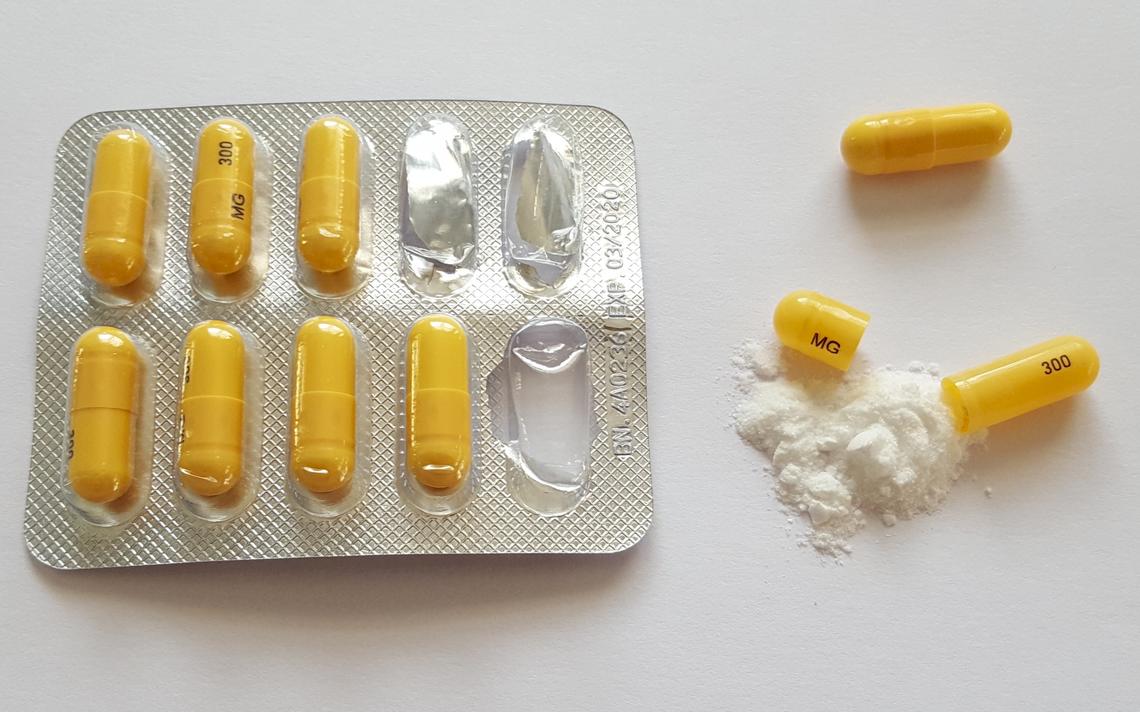
(a) Schedule II shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (b) Substances, vegetable origin or chemical synthesis. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including its salts: (1) Brivaracetam ((2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl]butanamide) (also referred to as BRV; UCB-34714; Briviact) (including its salts); (e) Stimulants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances having a stimulant effect on the central nervous system, including its salts, isomers (whether optical, position, or geometric), and salts of such isomers whenever the existence of such salts, isomers, and Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). The Uniform Controlled Substances Act, Title 37, Chapter 27, Idaho Code; (3-28-23) c. The rules of the Idaho State Board of Pharmacy; or (3-28-23) d. Any other applicable state or federal laws or regulations. (3-28-23) 02. Education, Training, and Experience. The act is consistent with licensee or registrant’s Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Idaho State Board of Pharmacy continued on page 4 2021 Summary of Statute Changes The following is a summary of the proposed substantive changes to the Idaho Code in the Pharmacy Practice Act, anticipated to become effective on July 1, 2021. The documents related to the Controlled Substances Act (CSA) and the Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Except that any combination or compound containing ephedrine, or any of its salts and isomers, or phenylpropanolamine or its salts and isomers, or pseudoephedrine, or any of its salts and isomers which is prepared for dispensing or over-the-counter distribution is not a controlled substance for the purpose of this section, unless such substance (c) It is unlawful for any person to possess a controlled substance unless the substance was obtained directly from, or pursuant to, a valid prescription or order of a practitioner while acting in the course of his professional practice, or except as otherwise authorized by this chapter. 37-2722. issuing, distributing, and dispensing of controlled substances. No person shall issue or dispense a prescription drug order for a controlled substance unless it is in compliance with applicable state and federal law and rules of the board. CHAPTER 27 UNIFORM CONTROLLED SUBSTANCES Boise, ID 83720-0054 P: 208-334-2475 TTY/TTD Call: 7-1-1 Website issues: E: lsoweb@lso.idaho.gov. Capitol Gift Shop; Initiate registration with the Idaho Prescription Drug Monitoring Program (PDMP) – Registration can be initiated at You may enter “Pending” when prompted to enter your Idaho license/registration number. PDMP registration is not required for Veterinarians or CET’s. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Justia U.S. Law U.S. Codes and Statutes Idaho Statutes 2014 Idaho Statutes Title 37 - FOOD, DRUGS, AND OIL Chapter 27 - UNIFORM CONTROLLED SUBSTANCES Article III Section 37-2722 - PRESCRIPTIONS. There Is a Newer Version of the I In October 2020, it became required by law to review a patient’s prescription drug history for the preceding 12 months from the prescription drug monitoring program (PMP) and evaluate the data for evidence of prescription misuse or diversion when prescribing an opioid analgesic or benzodiazepine listed in schedules II, III, or IV. In addition to the penalties prescribed in article IV of the uniform controlled substances act, any person shall be guilty of a felony who prescribes, dispenses, supplies, sells, delivers, manufactures or possesses with the intent to prescribe, dispense, supply, sell, deliver or manufacture anabolic steroids or any other human growth hormone for purposes of enhancing performance in an exercise 54-1753, and 54-1755, Idaho Code. ( ) 001. SCOPE. These rules regulate and control the manufacture, distribution, and dispensing of controlled substances within or into the state, pursuant to the Uniform Controlled Substances Act, Section 37-2715, Idaho Code; and regulate and control the practice of pharmacy, pursuant to the Idaho Pharmacy Act
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |