Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
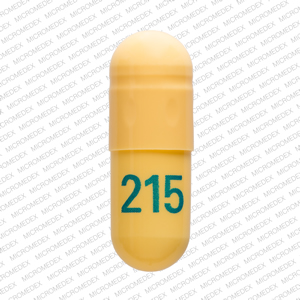
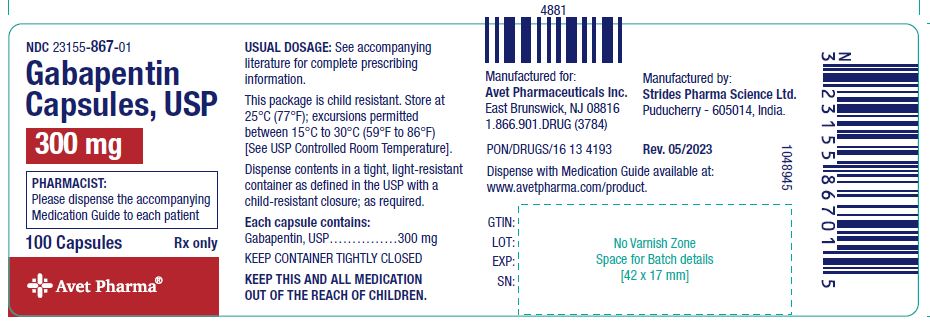
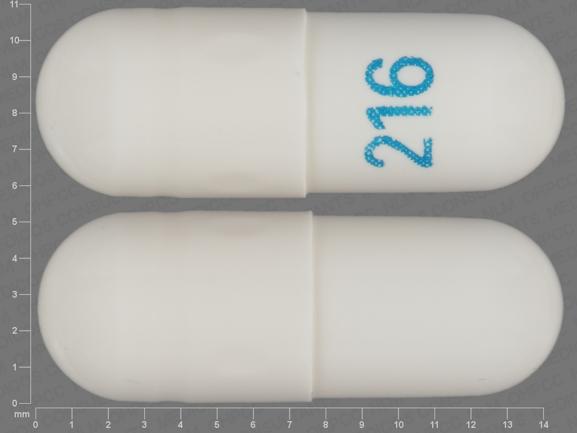
The short answer is, based on the language of Indiana Code § 35-48-7-11.3 and the guidance of the Professional Licensing Agency, it appears that any veterinarian that distributes, dispenses, or prescribes a controlled substance is required to register for INSPECT, and the IPLA requires that each practitioner INSPECT registrant have their own individual DEA License to register. Gabapentin is not a narcotic; however, according to the DEA, gabapentin has been increasingly documented as an illicit drug of abuse by police, in crime reports, and by U.S. poison control centers. Rates of diversion have also increased with gabapentin. Controlled Substances July 1, 2016: Pursuant to Indiana Code 25-26-13-4, ephedrine and pseudoephedrine, when dispensed via prescription, are each considered a "controlled substance" for the purposes of the INSPECT program. All prescription dispensations of these drugs must be reported to INSPECT. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. 11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. 2022 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5. 2021 Indiana Code Title 25. A prescription for a controlled substance under this section must be prescribed and dispensed in accordance with IC 25-1-9.3 and IC 25 (2) controlled substance or controlled substance analog (pure or adulterated), classified in schedule II, III, or IV; commits possession of a controlled substance, a Class A misdemeanor, except as provided in subsection (b). As used in this chapter, "controlled substance" has the meaning set forth in IC 35-48-1-9. The term includes gabapentin. As added by P.L.246-2019, SEC.21 and P.L.264-2019, SEC.10. However, due to a spike in gabapentin-related fatalities, Ohio, Kentucky and West Virginia have moved to list the drug as a controlled substance at the state level. Other states are recognizing the growing abuse problem with gabapentin and have, at the very least, mandated that it be included in their prescription drug monitoring programs. gabapentin has been increasingly encountered by law enforcement, documented in national crime lab reports, reported to poison control centers, and diverted for illicit use. In Indiana, gabapentin’s classification has been scrutinized due to its misuse potential. Although not classified under the federal Controlled Substances Act, the Indiana Board of Pharmacy designated gabapentin as a “drug of concern” in 2019. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Schedule III - The drug or other substance has a potential for abuse less than the drugs or other substances in schedules I and II. The drug or other substance has a currently accepted medical use in treatment in the United States. Abuse of the drug or other substance may lead to moderate or low physical dependence or high psychological dependence. July 1, 2016: Pursuant to Indiana Code 25-26-13-4, ephedrine and pseudoephedrine, when dispensed via prescription, are each considered a "controlled substance" for the purposes of the INSPECT program. All prescription dispensations of these drugs must be reported to INSPECT. As stipulated by IC 25-26-24-17, licensed dispensers throughout Indiana—and out-of-state (non-resident) pharmacies licensed to dispense drugs in Indiana—are required to submit controlled substance prescription data to INSPECT every twenty-four (24) hours. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. 2024 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5. ATTENTION - Effective July 1, 2016, pursuant to IC 35-48-2-8(c)(3), Butalbital, commonly known as Fioricet, is a Schedule III substance in the State of Indiana.As a result, Schedule III requirements in the Indiana Controlled Substances Act and associated administrative rules in 856 IAC 2 apply to its handling and dispensation.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |