Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
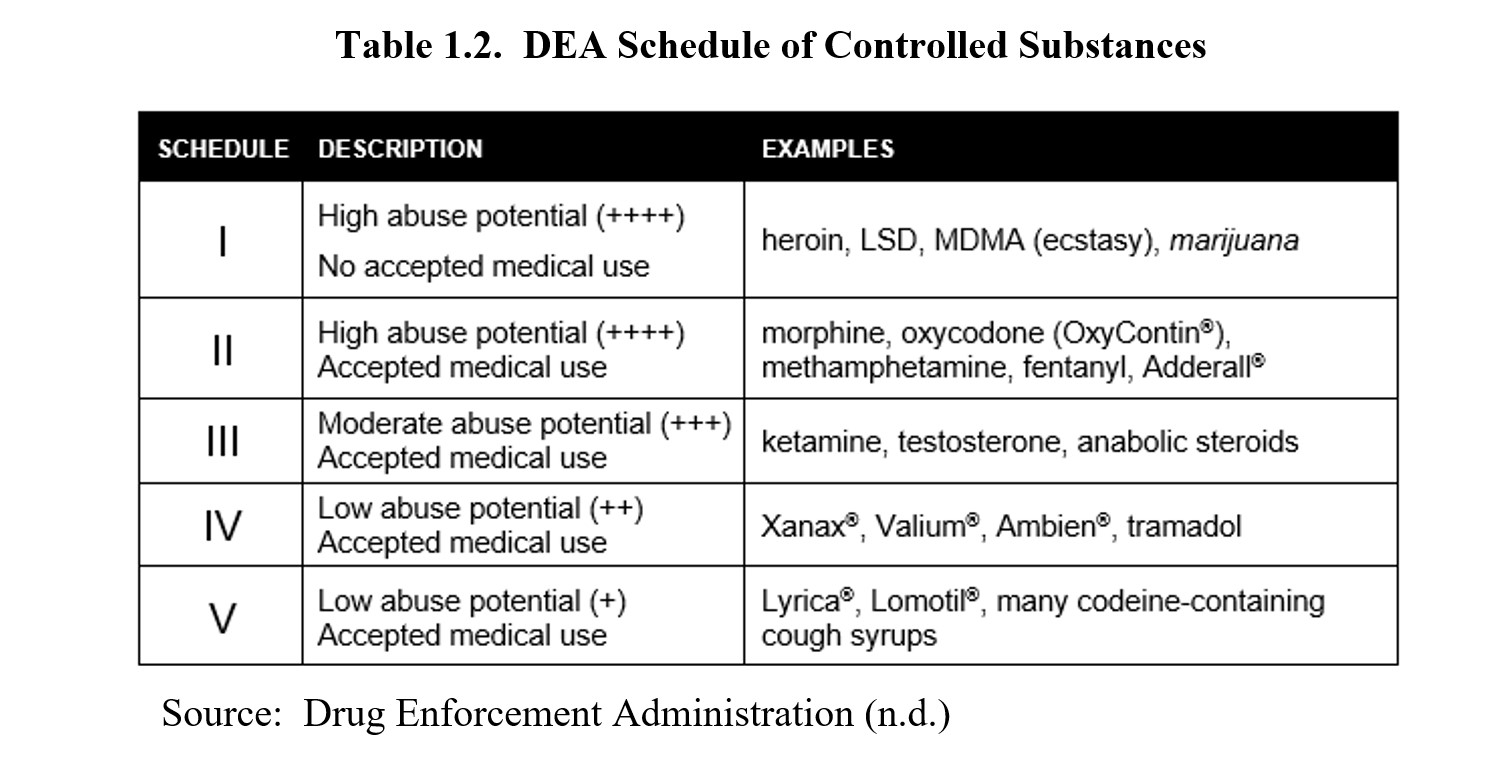
Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states, including Massachusetts. Learn why gabapentin is controlled, how it can be abused, and what states require reporting of prescriptions. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Massachusetts Board of Pharmacy and to all pharmacies in health facilities registered with the MA DPH that dispense federally controlled substances in Schedules II–V and gabapentin. In addition, the MA PMP reporting requirements apply to any pharmacy located in another state, commonwealth, district, or territory that delivers a prescription Pharmacies are required to submit data to the PMP for all dispensations of Schedule II-V medications and Gabapentin, a Schedule VI medication designated by the Commissioner as a drug of concern. Please note, the Veterans Administration (VA) is limited by statute to report only dispensations of federally controlled substances in Schedules II-V. Gabapentin, a medication commonly prescribed for nerve pain, is not currently classified as a controlled substance in Massachusetts. However, due to concerns about its potential for abuse, gabapentin is monitored by the state. schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. “The MCSR provides accountability for the manufacture, distribution, dispensing, possession, prescribing, and administering of controlled substances which, in Massachusetts, includes all prescription drugs.” The use of e-prescribing can reduce the risk of lost, stolen or forged prescriptions. An enrollment and security process must be completed to be an e-prescriber of controlled substances. The 2018 Massachusetts opioid legislation requires that all prescribers convert to secure electronic prescriptions (including Schedule II drugs) by 2020. Learn Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored To provide accountability for controlled substances, Massachusetts General Laws, Chapter 94C, section 7 and regulations of the Department of Public Health at 105 CMR 700.004 require every person who manufacturers, distributes, prescribes, administers, dispenses or possesses controlled substances to be registered with both the Department of Public Health and DEA if they utilize controlled Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky We register those who manufacture, distribute, dispense, possess, prescribe, or administer controlled substances. In Massachusetts, "controlled substances" includes all prescription drug products. Gabapentin and MassPAT The Massachusetts Prescription Awareness Tool (MassPAT) is an online tool utilized by authorized pro-viders that supports safe prescribing and dispensing of Schedule II-V controlled substances (CS). It is part of the Massachusetts Prescription Monitoring Program and requires pharmacies to report prescribing and dispensing For the purposes of establishing criminal penalties for violation of a provision of this chapter, there are established the following five classes of controlled substances: CLASS A Authorized prescribers and pharmacies must possess a Massachusetts Controlled Substance Registration. Electronic prescribing requirements are included in M.G.L. c. 94C, §§ 1, 17, 18, 20, and 23. MA Vol. 2, No. 3 Page 1 Identification Requirements for CS . Prescriptions. A pharmacy that dispenses federally designated con-trolled substances (CS) and Schedule VI prescription monitoring program (PMP) drugs (eg, gabapentin) is re-quired to check that the photo identification (ID) matches the customer taking possession of the prescription ÐÏ à¡± á> þÿ \ ^ þÿÿÿ Massachusetts (MA) Reporting 24,25 : The reporting requirement shall apply to every pharmacy that dispenses gabapentin, and to any pharmacy in another state, commonwealth, district or territory that delivers such drug to a person in Massachusetts.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |