Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
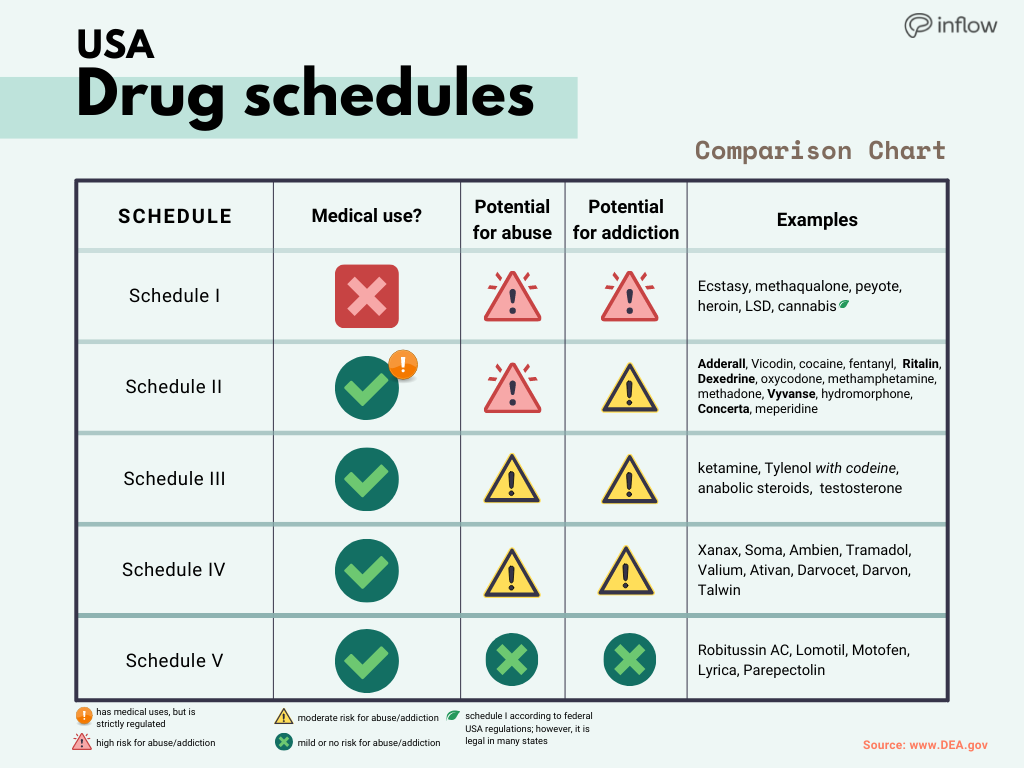
West Virginia; Several other states require gabapentin prescriptions to be monitored, allowing authorities to detect potential misuse: Campbell L, et al. (2021). Gabapentin controlled A prescription for a controlled substance other than one controlled in Schedule VI shall also contain the federal controlled substances registration number assigned to the prescriber. The prescriber's information shall be either preprinted upon the prescription blank, electronically printed, typewritten, rubber stamped, or printed by hand. A. It is unlawful for any person knowingly or intentionally to possess a controlled substance unless the substance was obtained directly from, or pursuant to, a valid prescription or order of a practitioner while acting in the course of his professional practice, or except as otherwise authorized by the Drug Control Act (§ 54.1-3400 et seq.). In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky The 2019 Virginia General Assembly recently passed a new bill that will classify gabapentin as a Schedule V substance starting July 1, 2019. Currently, gabapentin is classified as a Schedule VI drug of concern. This change in schedule will require gabapentin to be reported to the Virginia Prescription Monitoring Program as a schedule V substance. However, due to a spike in gabapentin-related fatalities, Ohio, Kentucky and West Virginia have moved to list the drug as a controlled substance at the state level. Other states are recognizing the growing abuse problem with gabapentin and have, at the very least, mandated that it be included in their prescription drug monitoring programs. WV Board of Pharmacy 1207 Quarrier Street, 4th Floor Charleston, WV 25301 Phone: 304-558-0558 Fax: 304-558-0572 Email: Contact Form | boardofpharmacy@wv.gov Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. "Controlled substance" means a drug, substance, or immediate precursor in Schedules I through VI of this chapter. "Controlled substance" does not include distilled spirits, wine, malt beverages, or tobacco as those terms are defined or used in Title 3.2 or Title 4.1. On July 1, 2019, veterinary establishments that possess gabapentin must take a complete and accurate inventory of this drug at the opening of business in accordance with §54.1-3404 and maintain compliance with 18VAC150-20-190 for a Schedule V controlled substance. In Virginia, gabapentin is classified as a Schedule V controlled substance under section 54.1-3454. This classification places it among substances with a lower potential for abuse relative to those in Schedules I through IV. As of September 2022, gabapentin was classified as a controlled substance in Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia. 6,7 Adding gabapentin to the list of controlled substances has required providers to have a Drug Enforcement Administrationregistration number to prescribe it, adding another layer of Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin, a Schedule V drug in the Commonwealth, had previously been considered a “drug of concern” in Virginia. The 2019 Virginia General Assembly passed HB2557, which classified gabapentin as a Schedule V controlled substance as of July 1, 2019. § 54.1-3446.Schedule I. The controlled substances listed in this section are included in Schedule I: 1. Any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, whenever the existence of these isomers, esters, ethers and salts is possible within the specific chemical designation: §60A-2-212. Schedule V. (a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. The Board may promulgate regulations designating specific drugs and substances, including any controlled substance or other drug or substance where there has been or there is the actual or relative potential for abuse, as drugs of concern. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following substances having a depressant effect on the central nervous system, including its salts: single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |