Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
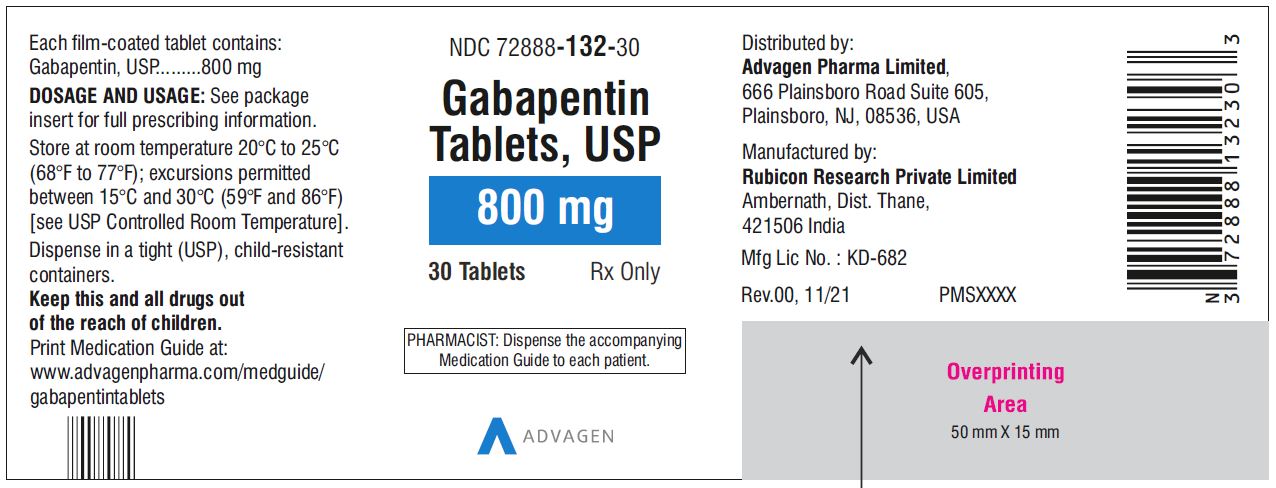 |  |
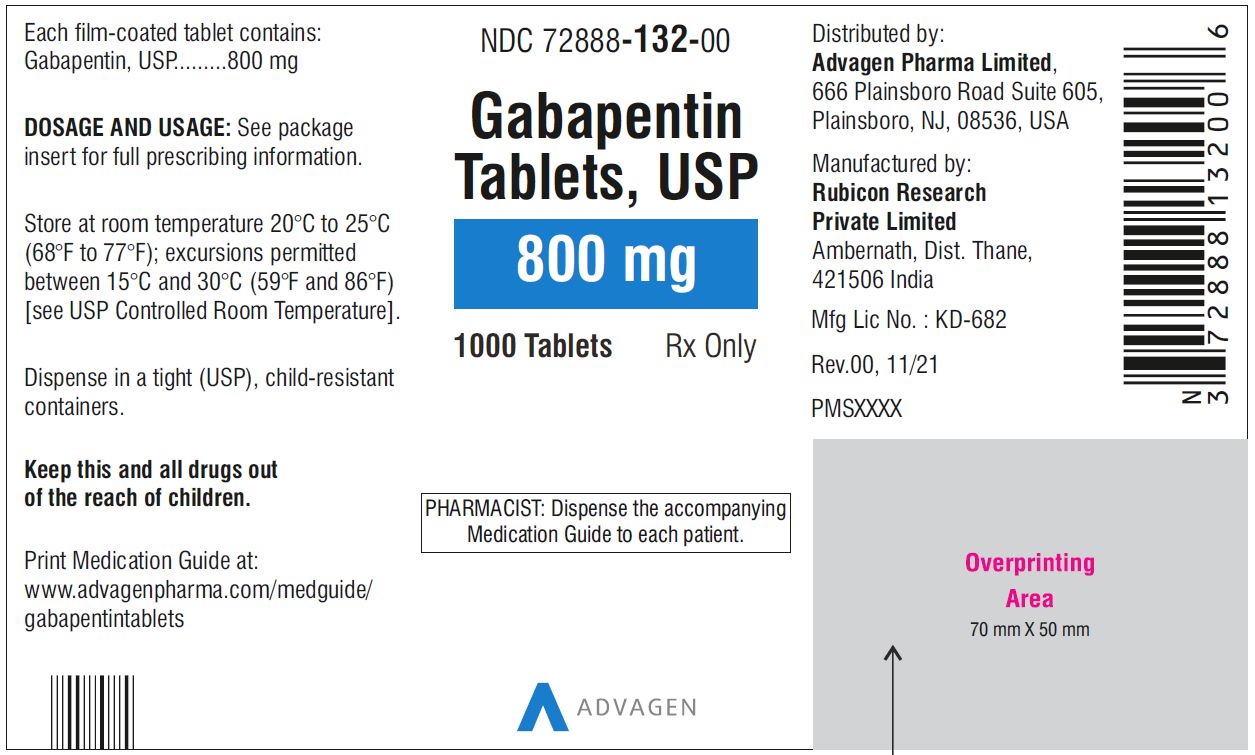
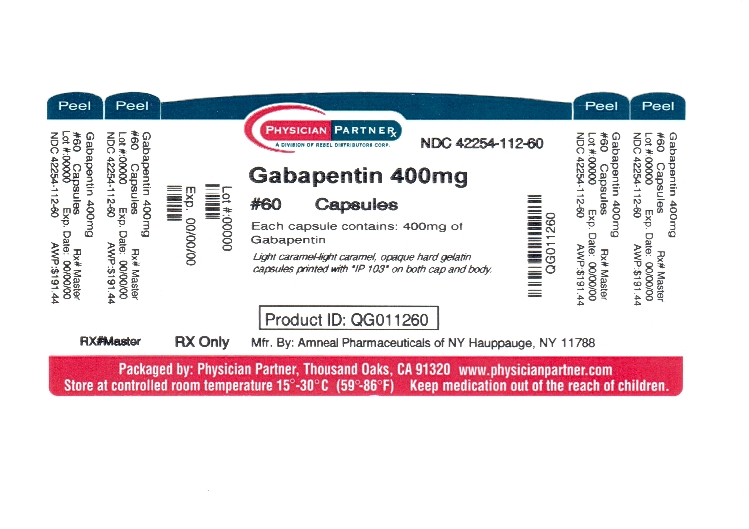
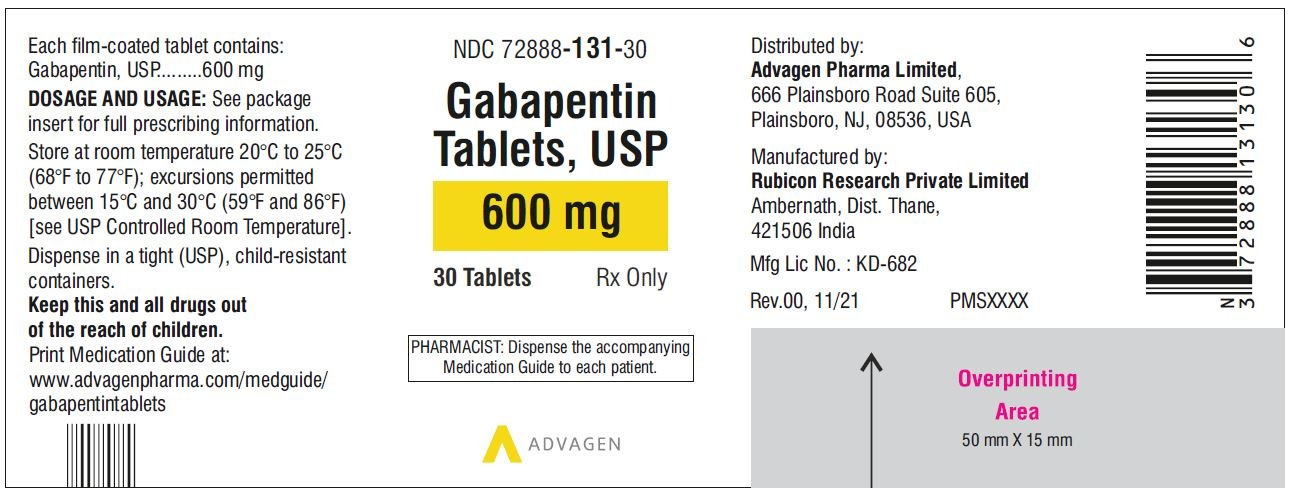
For adults, your gabapentin dosage varies depending on your medical conditions and which form you’re taking. The maximum dosage is 3,600 mg per day. For children, the dosage is based on age and body weight. Gabapentin is available as a lower-cost generic. But certain products are brand-only. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization Gabapentin Capsules, USP are indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. 2.1 Dosage for Postherpetic Neuralgia. In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. 2.1 Dosage for Postherpetic Neuralgia . In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). All Drugs; Human Drugs; Animal Drugs Neurontin ® (gabapentin) Capsules, Neurontin ® (gabapentin) Tablets, and Neurontin ® (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin Capsules are indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in INDICATIONS AND USAGE - Gabapentin capsules, USP, are indicated for: -management of postherpeticneuralgia in adults - -Adjunctive therapy in the treatment of partial onset seizures, with and DOSAGE AND ADMINISTRATION - Gabapentin capsules, USP are given orally with or without food. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Gabapentin is not appreciably metabolized in humans. Excretion. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. In elderly patients and patients with impaired renal function, plasma clearance is reduced. Gabapentin can be removed from plasma by hemodialysis. Gabapentin caused a marked decrease in neuronal synapse formation in brains of intact mice and abnormal neuronal synapse formation in a mouse model of synaptic repair. Gabapentin has been shown in vitro to interfere with activity of the α2δ subunit of voltage-activated calcium channels, a receptor involved in neuronal synaptogenesis. The Gabapentin capsules are a prescription medicine used to treat: Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance (see Special Populations: Patients With Renal Insufficiency
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |