Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 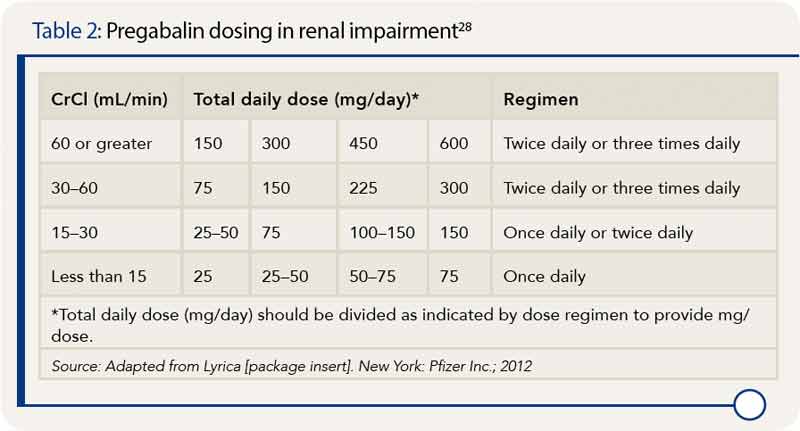 |
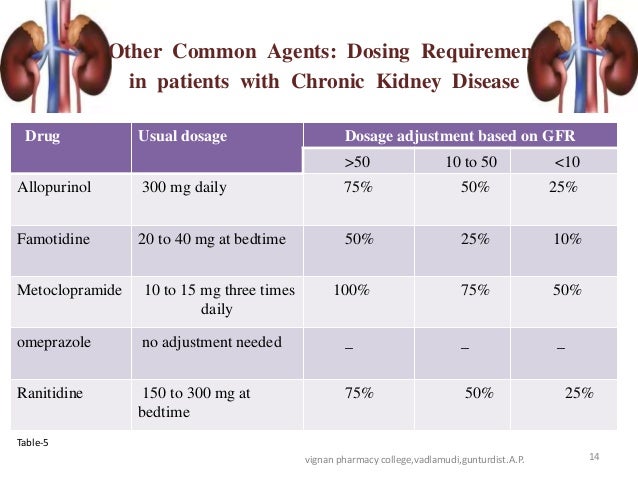
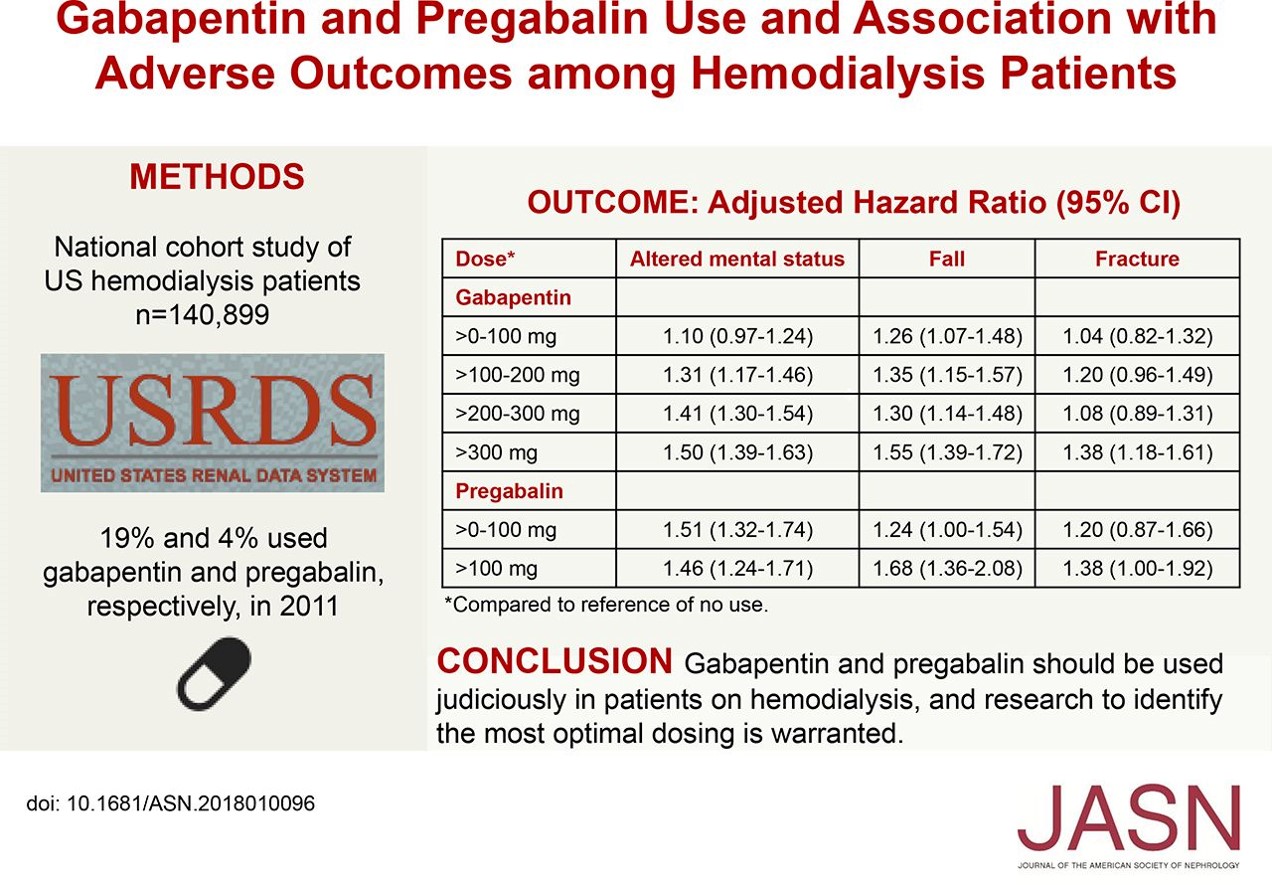
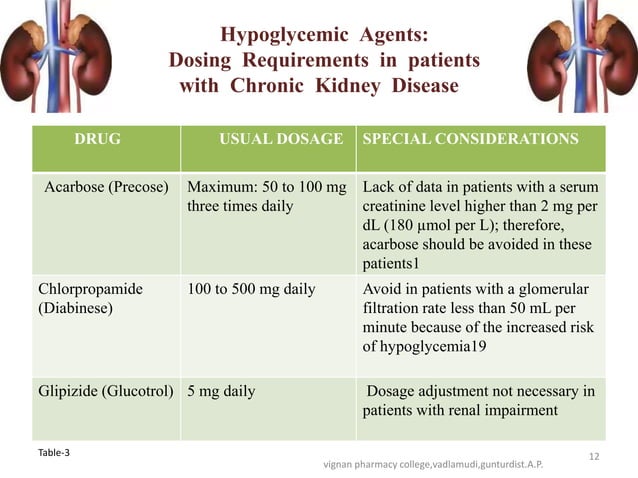
Usual initial gabapentin dose: 300mg q8h. Usual maintenance dose: 300-600mg q8h. Maximum dosage/day: 3600 mg. [15-29]: Dosage range: 200-700mg/day. [<15]: 100-300 mg/day. Use lower end of this range for CRCL <7.5 ml/min. TABLE 1. Gabapentin Dosage Based on Renal Function. TID = Three times a day; BID = Two times a day; QD = Single daily dose. a. Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. The short answer is: yes, gabapentin can be problematic for individuals with kidney failure and chronic kidney disease (CKD). While gabapentin is often prescribed for pain management, particularly nerve pain, and sometimes for seizures, its primary elimination pathway is through the kidneys. Discussion: Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. In patients with normal renal function, the maximum dose of gabapentin is 3600mg daily in divided doses. However, gabapentin is renally cleared and so the dose needs to be adjusted according to the GFR. For patients on dialysis, the recommended dose is 100-300mg post dialysis on dialysis days only. Loading dose of 300–400 mg in patients who have never received gabapentin. Maintenance dose of 200–300 mg after each HD : session and increase according to tolerability. Gabapentin toxicity in patients with chronic kidney disease is underrecognized. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Gabapentin dosing guidelines for adult with renal impairment are summarized in Table 3. Dosing guidelines for gabapentin immediate-release are also applicable for adolescents 12 years of age and older with renal impairment. Use: For the treatment of moderate-to-severe primary RLS in adults. Maximum dose: 2400 to 3600 mg/day; doses up to 2400 mg/day have been well tolerated in long-term studies; doses of 3600 mg/day have be used in a small number of patients for a relatively short duration and have been well tolerated. Affiliations 1 VA Tennessee Valley Healthcare System, Murfreesboro, Nashville, TN.; 2 Stratton VA Medical Center; Albany College of Pharmacy and Health Sciences, Albany, NY; Western New England University College of Pharmacy, Springfield, MA; Scientific and Clinical Affairs, Remitigate LLC, Delmar, NY, USA. Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided People with kidney disease. As gabapentinoids are predominantly excreted by the kidney, cautious starting doses and careful dose adjustments are required for people with acute or chronic kidney disease. When creatinine clearance is below 30 mL/minute, the half-lives of both gabapentin and pregabalin are prolonged. 8 Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Conclusion. Gabapentin’s apparent total clearance is 100 mL/min in adults with normal renal function, which is essentially equivalent to CrCl and does not suggest the involvement of tubular reabsorption. 1 Some evidence suggest that active tubular secretion mediated by organic cation transporter-1 (OCT-1) may play a role in gabapentin’s renal clearance. Patients receiving higher gabapentinoid doses with decreased kidney function may be at an increased risk of adverse effects (AEs), but limited evidence exists evaluating gabapentinoid dosing and AEs in this population. 4. Renal Dosing Recommendations. Mild Kidney Problems (CrCl 60-90 mL/min): Dose Adjustment: 900 - 3600 mg/day TID. How Often to Take: 3 times a day. Notes: Monitor for dizziness or double vision. Moderate Kidney Problems (CrCl 30-59 mL/min): Dose Adjustment: 400-1400 mg/day BID; How Often to Take: Twice a Day; Notes: Your doctor will decide the Accumulation can cause renal failure resulting in adverse effects. Formulations. Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. Pain is one of the most common and distressing symptoms among patients with chronic kidney disease (CKD) . The prevalence of pain has been associated with substantially lower health-related quality of life and greater psychosocial distress, insomnia, and depressive symptoms [ 2-9 ]. Notwithstanding, most reports of toxicities were associated with concentrations higher than 15 mg/L for gabapentin and concentrations higher than 13 mg/L for pregabalin, whereas individuals with normal renal function on maximum recommended dosing yielded concentrations of ~5–8 mg/L for gabapentin and 2.8–8.2 mg/L for pregabalin. 22–25 The
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |