Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 | 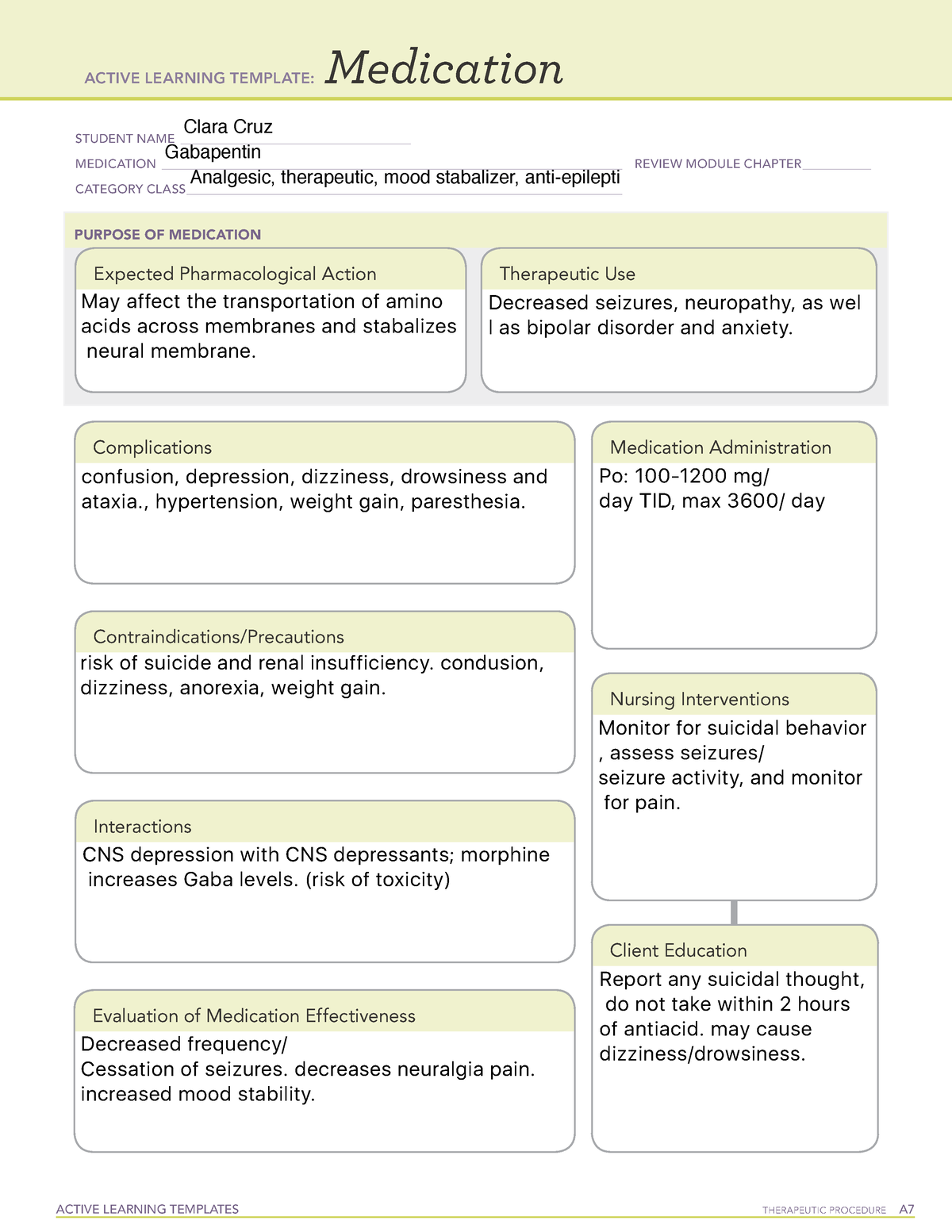 |
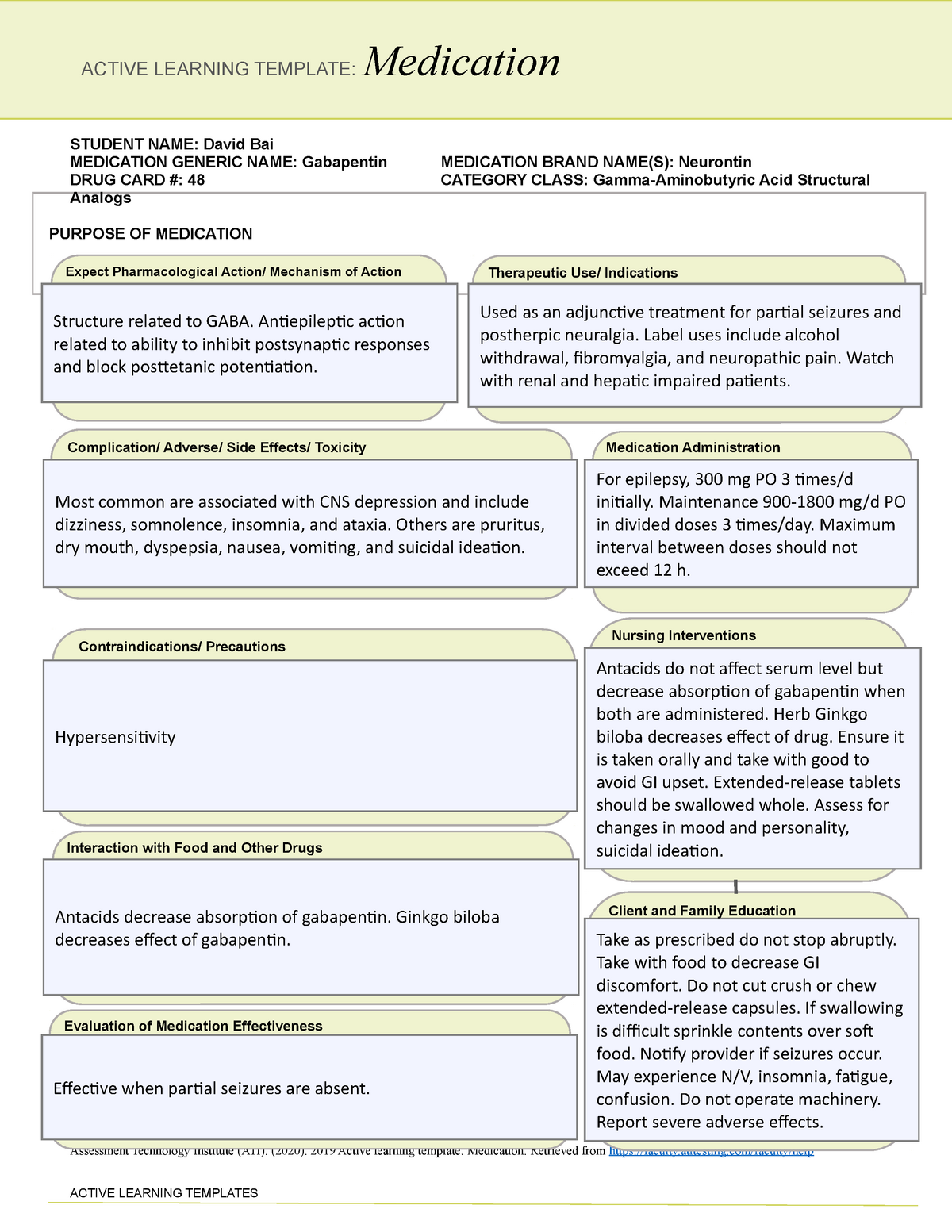
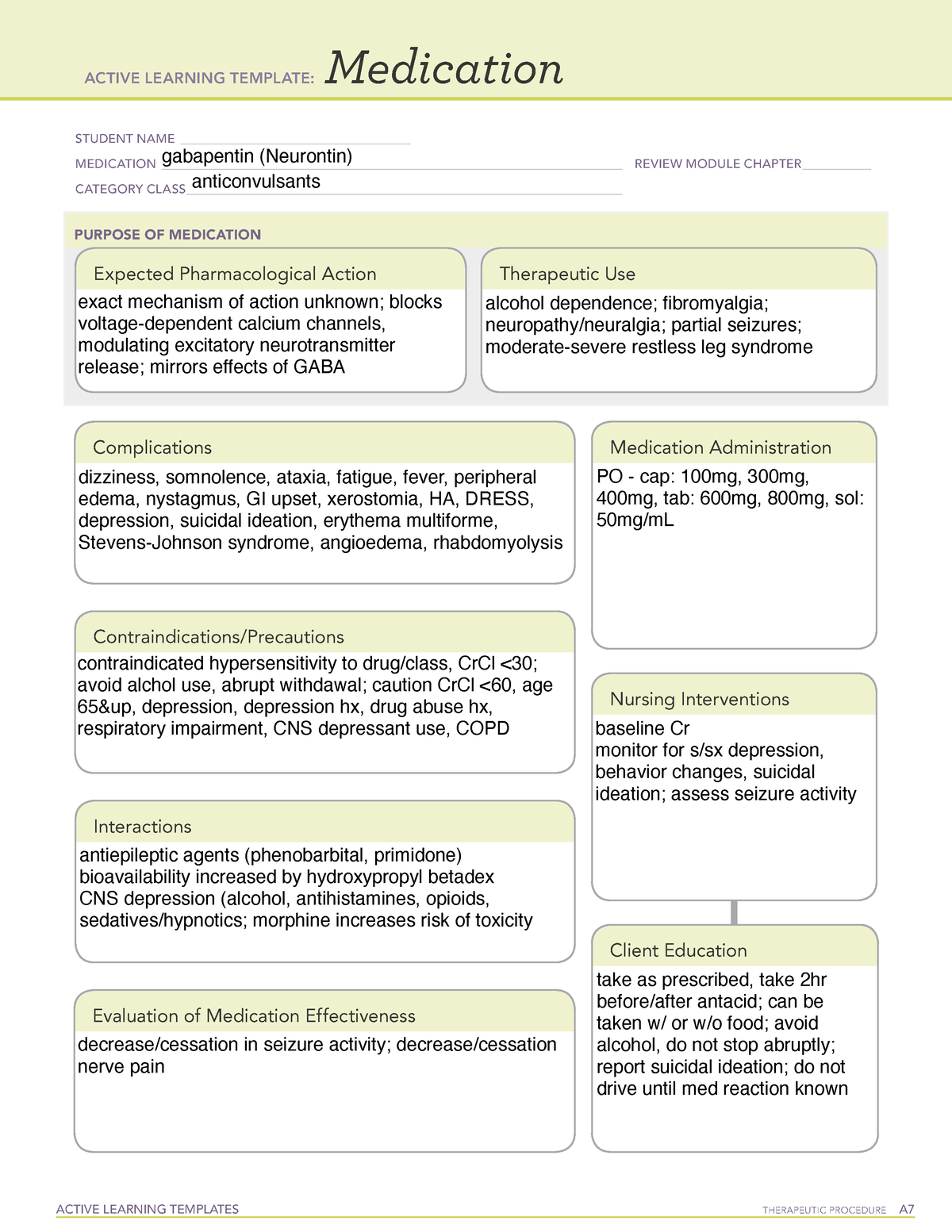
Pregabalin (Lyrica) and gabapentin (Neurontin) are both gabapentinoids, a class of nerve medication initially developed to treat epileptic seizures. Sales of Lyrica and Neurontin tripled a decade ago, when they were touted as safer alternatives to opioids and prescribed off-label for a variety of pain conditions. Last year the Advisory Council on the Misuse of Drugs recommended the drugs to be reclassified as class C drugs because of the risks of “illegal diversion and medicinal misuse”. Ministers have now accepted this recommendation and it will be implemented subject to consultation. What are gabapentin side effects? While gabapentin can be helpful in a number of circumstances, some of the common side effects associated with taking the drug as directed include drowsiness Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Generic name: gabapentin [ GA-ba-PEN-tin ] Drug class: Gamma-aminobutyric acid analogs. Medically reviewed by Philip Thornton, DipPharm. Last updated on Feb 21, 2025. Uses; Warnings; Before taking; Dosage; Side effects; Interactions; FAQ; What is Neurontin? Neurontin is an anti-epileptic drug, also called an anticonvulsant. It affects chemicals Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Most people are familiar with the term “class action lawsuit.” Many assume that all drug and device injury cases are class action lawsuits. But this is not always the case. A class action suit is when an individual or small group of plaintiffs acts as a leader for a larger group of injured people. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. Gabapentin is in a class of medications called anticonvulsants. Gabapentin treats seizures by decreasing abnormal excitement in the brain. Gabapentin relieves the pain of PHN by changing the way the body senses pain. It is not known exactly how gabapentin works to treat restless legs syndrome. Drug class: Gamma-aminobutyric acid analogs. Medically reviewed by Drugs.com. Last updated on Feb 26, 2025. The investigators advised that the growth in use was primarily driven by gabapentin, and no increase in pregabalin users was detected after 2008 or after generic availability in 2019. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it. Why does gabapentin’s drug class vary from state to state? Although gabapentin isn’t controlled federally, some states have listed it as a controlled substance and therefore regulate its use. Columbus, OH—Gabapentinoids are prescribed primarily for off-label indications with limited evidence to support their benefits, but that has not stopped the steady increase in use of the drug class, according to a new study. Gabapentin is frequently combined with other substances for the purpose of potentiating the effects of the drugs or achieving a “high.” Studies have identified various substances that are commonly abused in combination with gabapentin, including alcohol, opioids, benzodiazepines, antidepressants, and other CNS depressants 13,14,15. Having originally been developed as an anti-seizure medication, gabapentinoids include gabapentin and pregabalin, which are now prescribed primarily for neuropathic pain, seizures and anxiety, but also for fibromyalgia, restless legs syndrome and complications of MS (Chan et al, 2023). Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. The FDA has previously required medication guides and labeling updates for antiepileptic drugs including gabapentin. Latest Neurontin Lawsuit Updates. January 28, 2025 – Schmidt&Clark law firm continues to investigate Neurontin-induced Stevens-Johnson Syndrome cases across all 50 states, offering free consultations to affected patients. Texas Prior Authorization Program Clinical Criteria Gabapentin . October 30, 2023 Revised January 15, 2025 Copyright © 2025 Health Information Designs, LLC 10
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |