Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
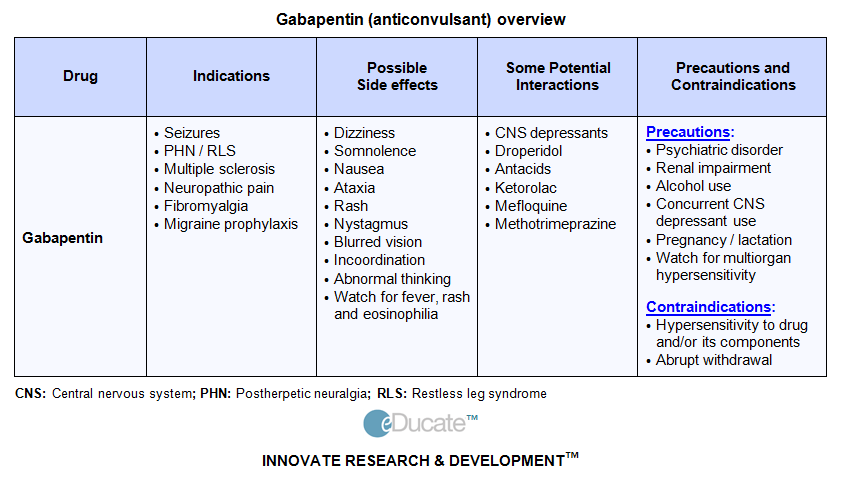 |  |
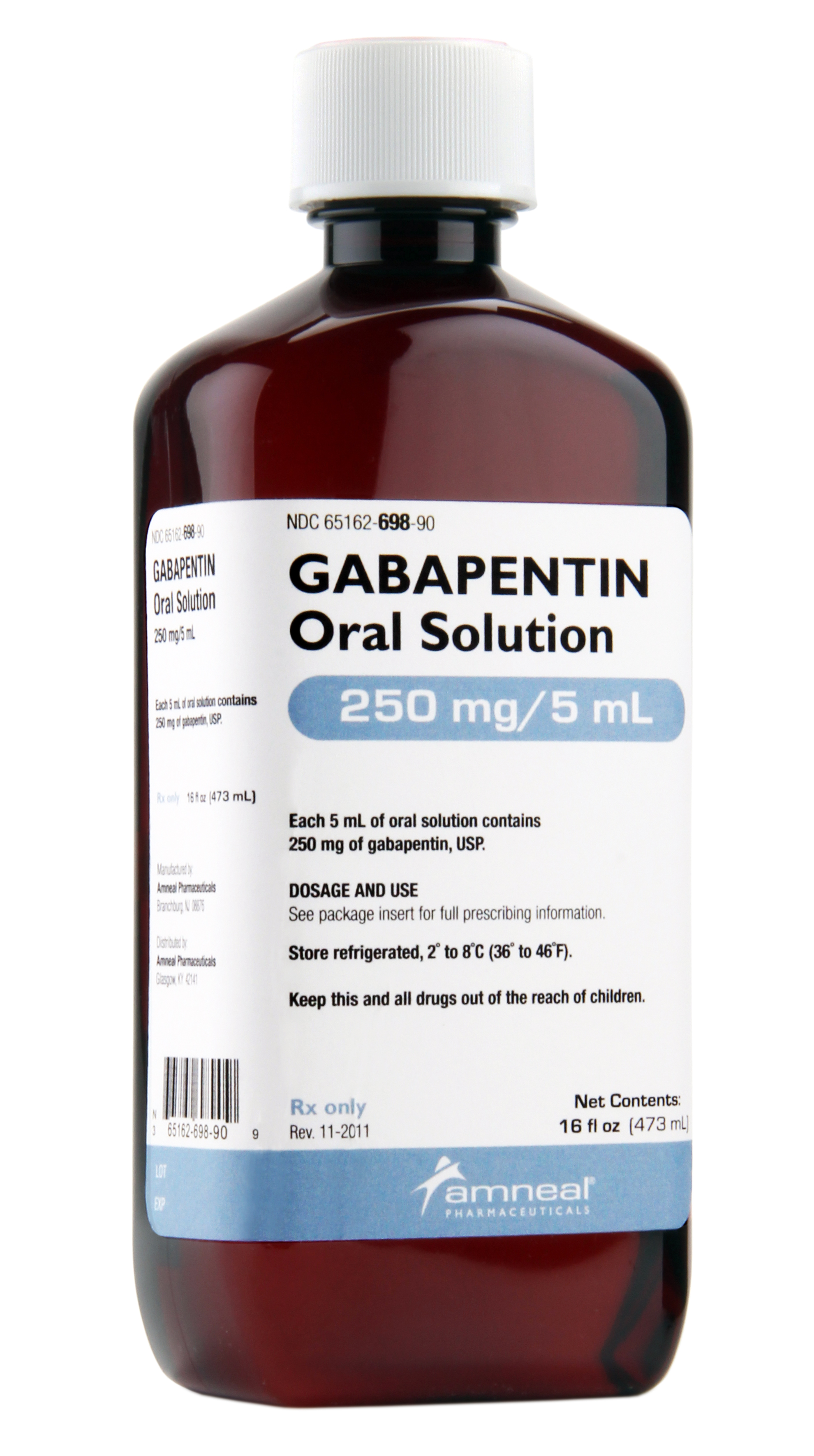
From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Gabapentin is a prescription drug most commonly prescribed to relieve nerve pain following shingles in adults, treating the pain of post herpetic neuralgia. Gabapentin belongs to a class of drugs known as anti-seizure drugs. Take gabapentin by mouth as directed by your doctor, usually once a day with the evening meal. Gabapentin is an anti-epileptic drug, also called an anticonvulsant. Drug class: Gamma-aminobutyric Your dose needs may change if you switch to a different 9 This bill adds gabapentin to the list of controlled substances. 10 Highlighted Provisions: 11 This bill: 12 < adds gabapentin to Schedule V of the list of controlled substances; and 13 < makes technical and conforming changes. 14 Money Appropriated in this Bill: 15 None 16 Other Special Clauses: 17 None 18 Utah Code Sections Affected: 19 AMENDS: We compared two interventions including (1) class V scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence It is not "Scheduled" Federally or in State Statute, it is still a legend drug regulated by the FDA by general Rx order. At this time there is no change or new regulation with prescribing gabapentin. The only change in regulation for gabapentin is that it must be to report to the Controlled Substance Database. Gabapentin is in a class of medications called anticonvulsants. What are the brand names of gabapentin? Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Generic name: gabapentin [ GA-ba-PEN-tin ] Drug class: Gamma-aminobutyric acid analogs. Medically reviewed by Philip Thornton, DipPharm. Last updated on Feb 21, 2025. Uses; Warnings; Before taking; Dosage; Side effects; Interactions; FAQ; What is Neurontin? Neurontin is an anti-epileptic drug, also called an anticonvulsant. It affects chemicals Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Last year the Advisory Council on the Misuse of Drugs recommended the drugs to be reclassified as class C drugs because of the risks of “illegal diversion and medicinal misuse”. Ministers have now accepted this recommendation and it will be implemented subject to consultation. The UK government announced last week that the medicines pregabalin and gabapentin will be reclassified as class C under the Misuse of Drugs Act 1971. The change will take place in April 2019. Class C is the third in the government’s three-tier system for categorising controlled substances. • The Home Office reclassified the prescription drugs pregabalin and gabapentin as class C drugs in April 2019 after concerns about misuse and addiction; • The change in law made it illegal to possess the drugs without a prescription, and supply or sell them to others; Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations ‘In April 2019, following a public consultation, as well as advice from the Advisory Council of the Misuse of Drugs, gabapentinoids were reclassified as Class C controlled substances and scheduled under the Misuse of Drugs Regulations 2001’. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |