Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
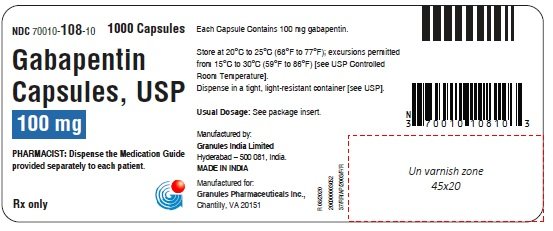
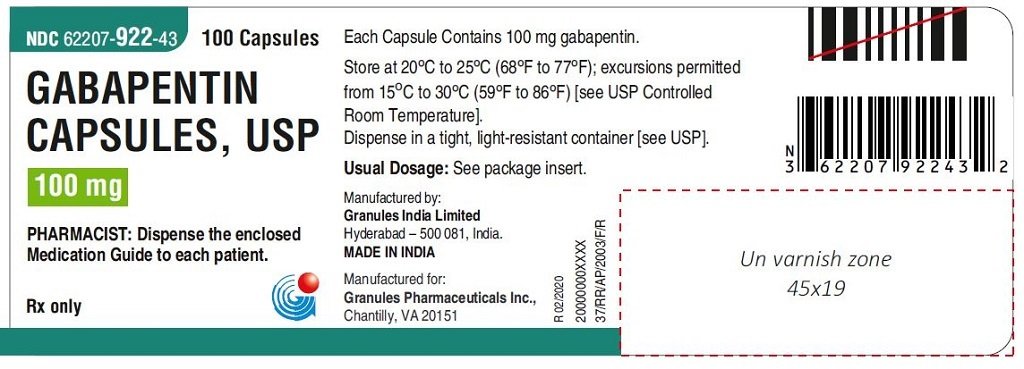
Gabapentin is a prescription medication that was approved by the U.S. Food and Drug Administration in 1993 as a treatment for epilepsy. It works by binding to a type of calcium channel in nerve To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Drug Review Package. 10903 New Hampshire Avenue Silver Spring, MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA Gralise FDA Approval History. FDA Approved: Yes (First approved January 28, 2011) Brand name: Gralise Generic name: gabapentin Dosage form: Extended Release Tablets Previous Name: DM-1796 Company: Depomed, Inc. Treatment for: Postherpetic Neuralgia Finally, I downloaded the most recently updated US Food and Drug Administration (FDA)-approved product insert for gabapentin, which gained FDA approval in 1993, and its more potent successor, pregabalin, which was approved by the FDA in 2004. 7,8 Both drugs are available in generic formulations, and a 90-day supply of an average dose of either History. In 1993, the FDA approval of Neurontin, the original branded gabapentin, was for use as an adjunctive medication to control partial seizures. 9 Over the next several years, the manufacturer, Parke-Davis, a subsidiary of Warner-Lambert, engaged in a large marketing campaign to increase off-label prescribing of Neurontin for pain. 4 By U.S. Food and Drug Administration Search Menu; Search FDA Submit cat, horse FDA-2018-N-4626-0032 FDA-2018-N-4626-0051 FDA-2018-N-4626-0084 Gabapentin/trazodone HCl dog, cat FDA-2018-N History. Gabapentin was originally discovered over 40 years ago by the Japanese, who initially were looking for an antispasmodic or muscle relaxant. It was later sold to Parke-Davis (Warner-Lambert, which merged with Pfizer in 2000), who discovered effectiveness of gabapentin for treating epileptics. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Gabapentinoid products include gabapentin, which is marketed under the name Neurontin and Gralise, as well as generics; gabapentin enacarbil, a prodrug of gabapentin which is marketed as Horizant Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. Approval by the U.S. Food and Drug Administration followed in December 1993, also for use as an adjuvant (effective when added to other antiseizure drugs) medication to control partial seizures in adults; that indication was extended to children in 2000. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Specifications will be determined upon review of the data submitted in the application. Conduct testing on both test and reference products accordingly, and provide data on individual unit, means, Neurontin® (gabapentin) capsules, Neurontin® (gabapentin) tablets, and Neurontin® (gabapentin) oral solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Background & History. First developed in the 1970s, gabapentin was approved by the FDA in 1993 for use in the United States. It has been marketed under the name Neurontin, although other brand names exist. Gabapentin is structurally related to gamma-aminobutyric acid (GABA), a neurotransmitter in the brain. Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |