Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 | 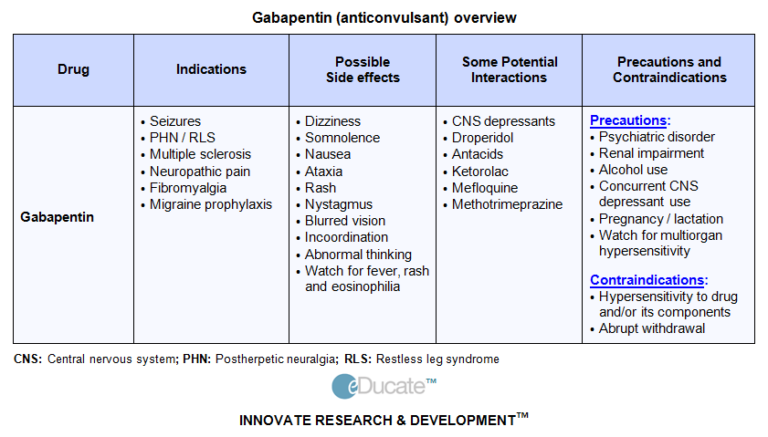 |
Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Indiana’s classification of controlled substances is governed by the Indiana Code Title 35, Article 48, aligning with the federal Controlled Substances Act. The state categorizes drugs into five schedules based on potential for abuse, accepted medical use, and safety under medical supervision. 2024 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5. As stipulated by IC 25-26-24-17, licensed dispensers throughout Indiana—and out-of-state (non-resident) pharmacies licensed to dispense drugs in Indiana—are required to submit controlled substance prescription data to INSPECT every twenty-four (24) hours. Prescription data submissions must be submitted through the PMP Clearinghouse. Important notice regarding changes to the law affecting prescribing practitioners: Effective July 1st, 2019: pursuant to House Enrolled Acts 1246 and 1542, the Indiana scheduled prescription electronic collection and tracking (INSPECT) program will begin monitoring and tracking the prescribing and dispensation of Gabapentin. In Indiana, gabapentin’s classification has been scrutinized due to its misuse potential. Although not classified under the federal Controlled Substances Act, the Indiana Board of Pharmacy designated gabapentin as a “drug of concern” in 2019. Information System (NFLIS) Drug database collects scientifically verified data on drug items and cases submitted to and analyzed by participating federal, state and local forensic , drug laboratories. NFLIS-Drug received 3,614 reports of gabapentin in 2019; 3,348 in 2020; 3,128 Gabapentin can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you: • However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Taking gabapentin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before taking opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Tell your doctor about all your current medicines. Many drugs can affect gabapentin, especially: naproxen; Schedule I Drugs: Examples include heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3, 4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote. A complete list of current federal controlled substances, their Schedule, and whether they are a narcotic can be found the website of U.S. Department of Justice. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence As used in this chapter, "controlled substance" has the meaning set forth in IC 35-48-1-9. The term includes gabapentin. As added by P.L.246-2019, SEC.21 and P.L.264-2019, SEC.10. Disclaimer: These codes may not be the most recent version. Indiana may have more current or accurate information. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |