Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
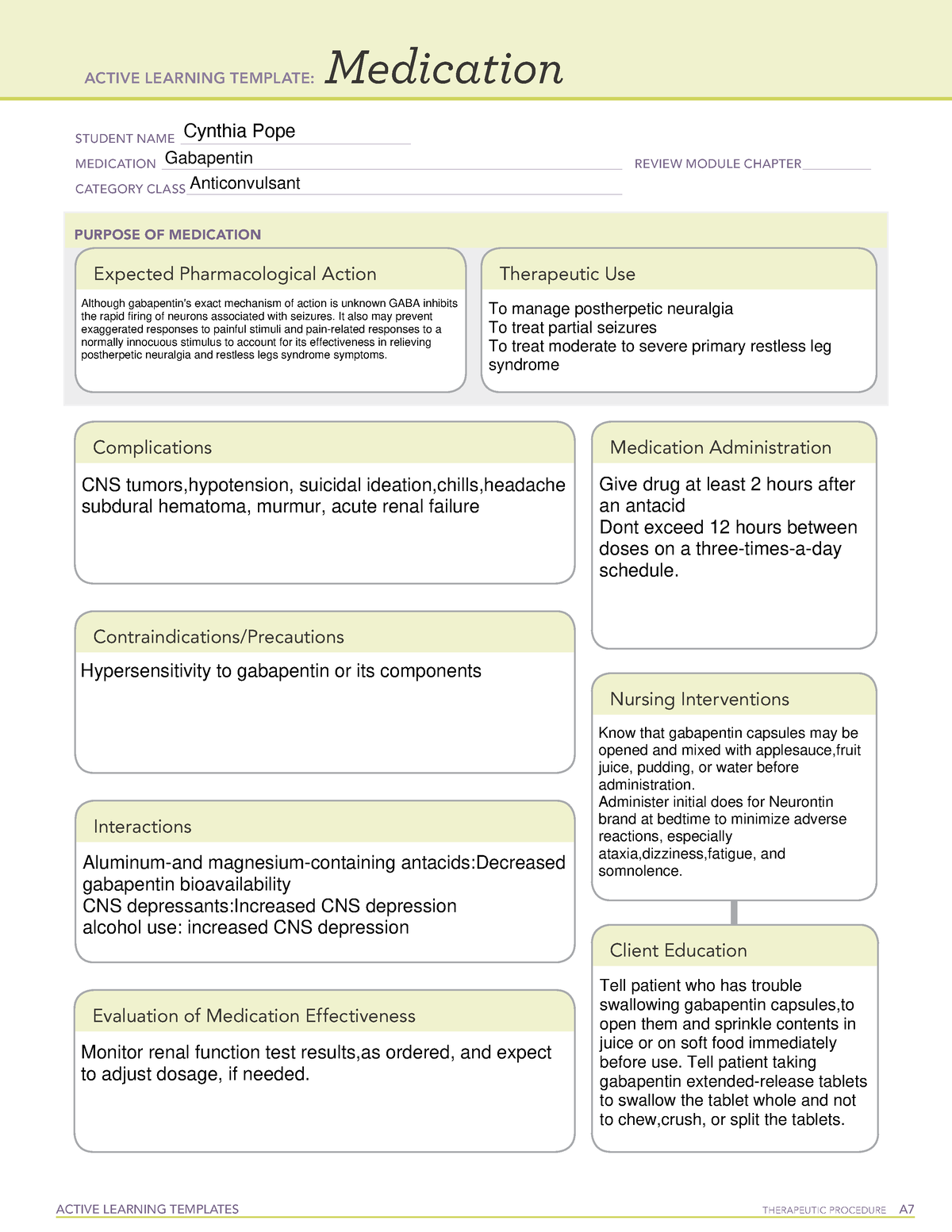 |  |
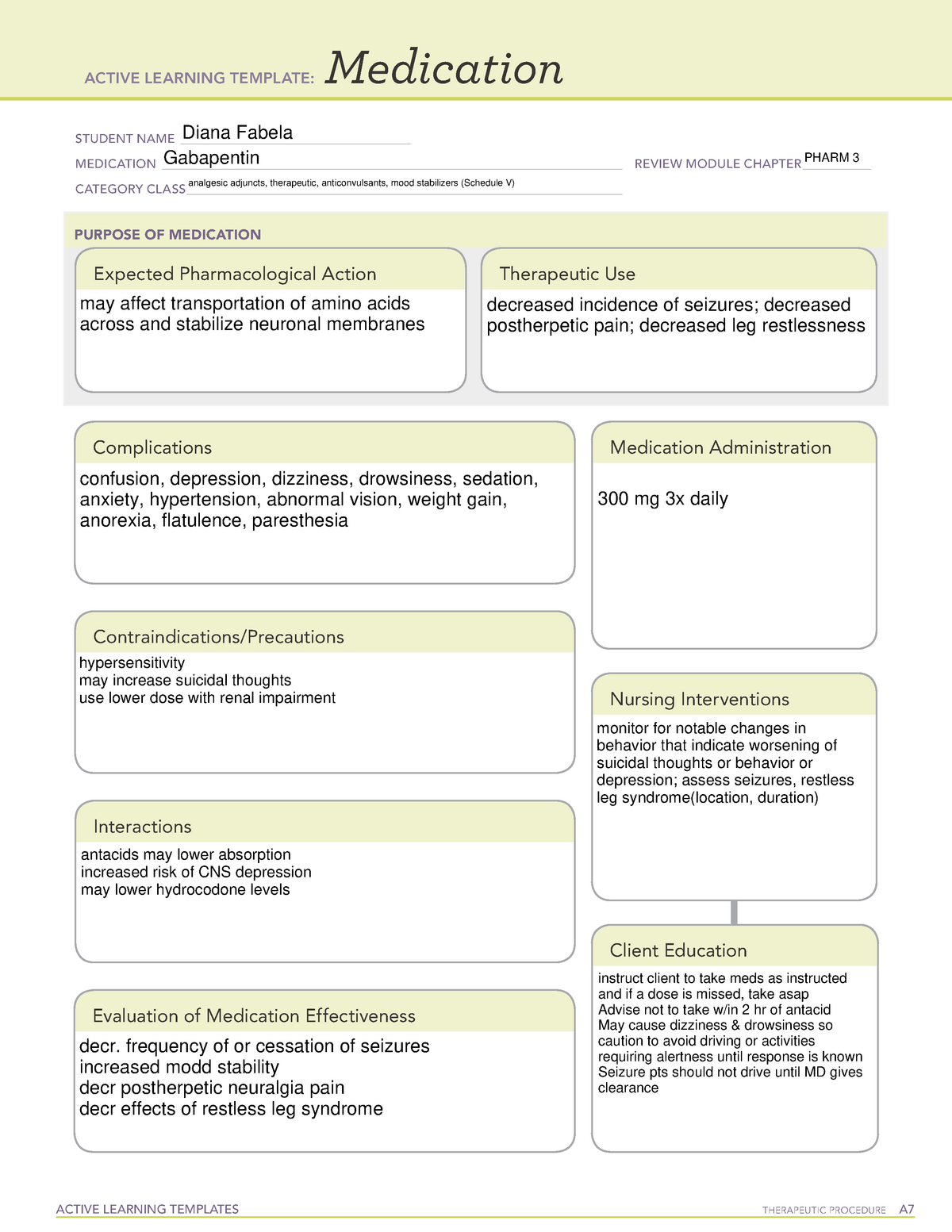
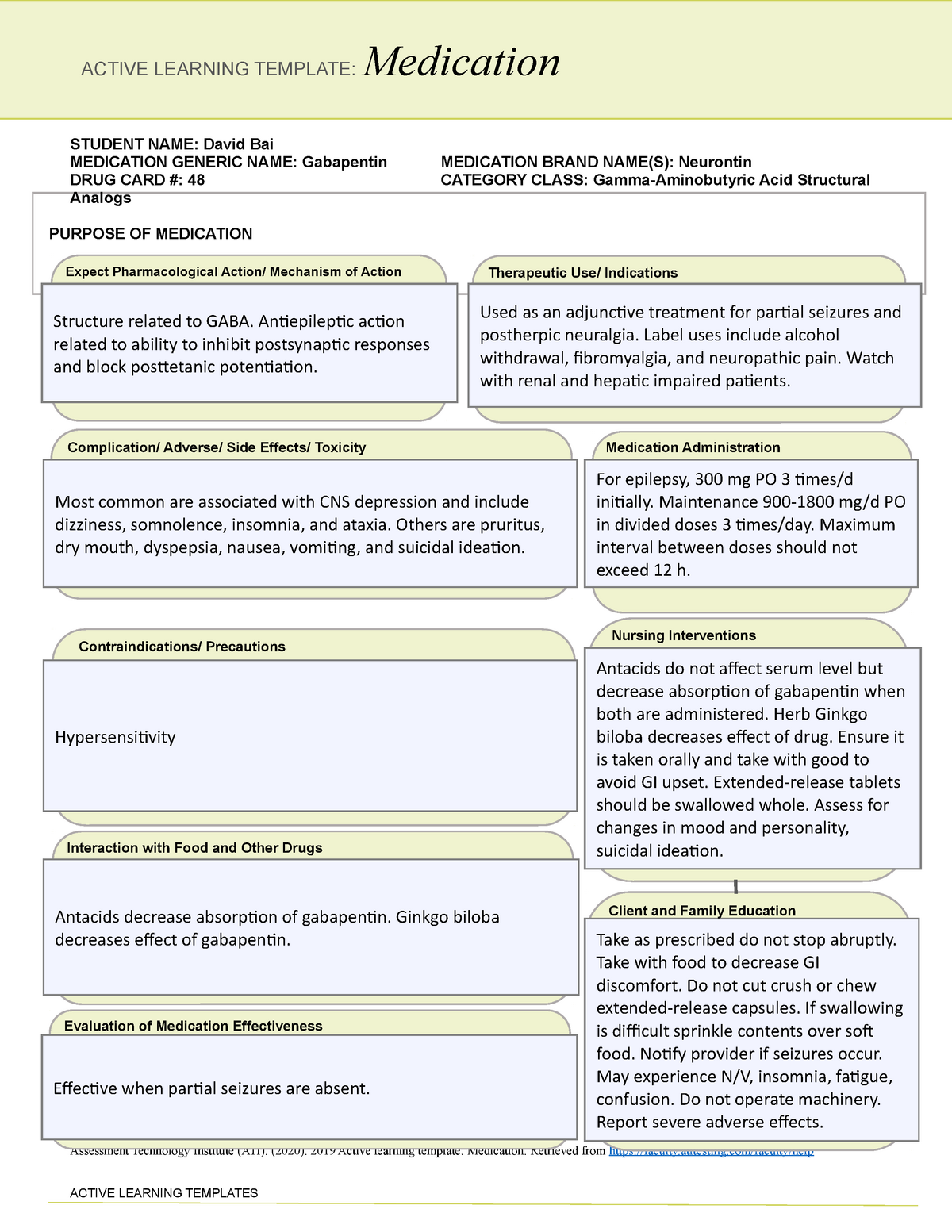
Massachusetts (MA) Reporting 24,25 : The reporting requirement shall apply to every pharmacy that dispenses gabapentin, and to any pharmacy in another state, commonwealth, district or territory that delivers such drug to a person in Massachusetts. ''Extended-release long-acting opioid in a non-abuse deterrent form'', a drug that is: (i) subject to the United States Food and Drug Administration's extended release and long-acting opioid analgesics risk evaluation and mitigation strategy; (ii) an opioid approved for medical use that does not meet the requirements for listing as a drug with Use this button to show and access all levels. This page links to an unofficial version of each section of G.L. c. 94C. Please note that sections that appear missing have been either reserved for future use or repealed. Added by St.2023, c.28, § 42, effective July 1, 2023. For the purposes of establishing criminal penalties for violation of a provision of this chapter, there are established the following five classes of controlled substances: Pharmacies are required to submit data to the PMP for all dispensations of Schedule II-V medications and Gabapentin, a Schedule VI medication designated by the Commissioner as a drug of concern. Please note, the Veterans Administration (VA) is limited by statute to report only dispensations of federally controlled substances in Schedules II-V. associated with gabapentin use could potentially decrease with statewide expansion of these programs. The use of therapeutic drug monitoring in routine clinical practice to optimize gabapentin’sefficacy and safety has not identified a relationship between gabapentin levels and Key Points Background: Gabapentin is an anticonvulsant used to Class C drugs are defined under Chapter 94C, Section 31 of the Massachusetts General Laws. This category includes substances that have a potential for abuse but are considered to have a lower risk compared to Class A and B drugs. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. While gabapentin is NOT considered as a controlled substance on a federal level, there are some states that reclassified the drug as a controlled substance. The five states that have reclassified gabapentin as a controlled substance are Kentucky, Virginia, West Virginia, Michigan, and Tennessee. Federal regulations . Title 21 CFR:. Chapter I: Food and Drug Administration, Department of Health and Human Services. Subchapter C - Drugs: General, Parts 200-299 New: 21 CFR part 202 Prescription drug advertising Taking gabapentin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before taking opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Tell your doctor about all your current medicines. Many drugs can affect gabapentin, especially: naproxen; These drugs are considered to have a lower potential for abuse compared to Class A, B, and C drugs, but they are still regulated under Massachusetts law. Despite the legalization of recreational marijuana in Massachusetts, possessing large amounts or selling marijuana without proper licensing remains illegal. Information System (NFLIS) Drug database collects scientifically verified data on drug items and cases submitted to and analyzed by participating federal, state and local forensic , drug laboratories. NFLIS-Drug received 3,614 reports of gabapentin in 2019; 3,348 in 2020; 3,128 Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. ÐÏ à¡± á> þÿ \ ^ þÿÿÿ 2006 Massachusetts Code - Chapter 94C — Section 31. Classes of controlled substances; establishment of criminal penalties forviolations of this chapter. Section 31. For the purposes of establishing criminal penalties for violation of a provision of this chapter, there are established the following five classes of controlled substances: CLASS A For the purposes of establishing criminal penalties for violation of a provision of this chapter, there are established the following five classes of controlled substances: CLASS A. the Massachusetts Prescription Monitoring Program and requires pharmacies to report prescribing and dispensing information for all drugs in Schedules II-V and those drugs in Schedule VI that have been designated as “ad-ditional drugs.” This information is then used to provide more complete information to prescribers, dispensers, and Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Schedule VI drugs consist of all prescription drugs which are not included in Schedules II-V. This is a special Massachusetts schedule (MGL 94C, 105 CMR 720.002). MGL 94C Section 3 states: “SCHEDULE VI.— (A) The substance is a prescription drug; and (B) Said prescription drug has not been included in Schedules I through V.”
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |