Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
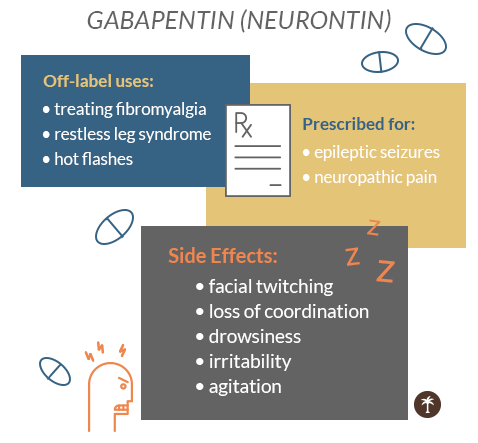
Gabapentin, a drug viewed as an alternative to opioids, is being abused across Ohio, experts and state officials warn. The misuse could lead the state to reclassify the drug. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin has not been reclassified as a controlled substance, but it is being added to the Board’s list of drugs reportable to OARRS following increased reports of misuse, abuse, and concomitant abuse of gabapentin nationwide.1. Gabapentin is a prescription medication originally approved to treat seizure and nerve pain disorders. Since its approval, gabapentin has become the drug of choice for many disorders outside of its labeled indication. Gabapentin is generally seen to be a helpful medication with few risks, but it’s still possible for abuse to occur. Unless specifically exempted or excluded under federal drug abuse control laws or unless listed in another schedule, any drug product in finished dosage formulation that has been approved by the United States food and drug administration that contains cannabidiol (2-[1R-3-methyl-6R-(1- methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol at least one preferred drug in the same class, if available NON-PREFERRED CRITERIA: • Must have had an inadequate clinical response of at least 30 days with at least two preferred drugs in the same class, if available P 0.1% BvG apraclonidine ALPHAGAN P 0.15% BvG brimonidine 0.1%, 0.15% rimonidine 0.2% IOPIDINE BETA BLOCKERS betaxolol BETIMOL Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. *These are drug products which: (1) may be dispensed only upon a prescription issued by a practitioner and, (2) contain controlled substances but have been specifically excepted from the controlled substances schedules. (Title 21, CFR 1308.31.) Accordingly, these drugs are legally classified as dangerous drugs in Ohio. Rx-Prescription Drugs. Information System (NFLIS) Drug database collects scientifically verified data on drug items and cases submitted to and analyzed by participating federal, state and local forensic , drug laboratories. NFLIS-Drug received 3,614 reports of gabapentin in 2019; 3,348 in 2020; 3,128 77 South High Street, 17th Floor, Columbus, Ohio 43215 T: (614) 466.4143 | F: (614) 752.4836 | contact@pharmacy.ohio.gov | www. pharmacy.ohio.gov. Controlled Substance Reference Table Annual Review Completed for all Drug Entries on 9-15-2019 . Please be advised that the information contained in this table is compiled solely Stats PDMP Interactive Data Tool. Ohio's prescription drug monitoring program, known as the Ohio Automated Rx Reporting System (OARRS), collects information on the distribution of prescription controlled substances and two non-controlled drugs, gabapentin and naltrexone, to Ohio patients. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Neurontin® Widely Sought for Illicit Use. OSAM-o-Gram. February 2017. Neurontin® (gabapentin) is an anticonvulsant medication which is also used to treat nerve pain in adults. The U.S. Centers for Disease Control and Prevention lists gabapentin as an appropriate non-opioid treatment for chronic pain, recommending the drug as a first-line care, the Ohio Board of Pharmacy created Ohio’s Prescription Drug Monitoring Program (PDMP), known as the Ohio Automated Rx Reporting System (OARRS). OARRS collects information on all outpatient prescriptions for controlled substances and gabapentin dispensed by Ohio-licensed pharmacies and personally furnished by licensed prescribers in Ohio. Gabapentin is in a class of medications called anticonvulsants. What are the brand names of gabapentin? Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. In 2020, a federal court case in Ohio examined gabapentin’s role in the opioid crisis. While the court did not reclassify gabapentin, it emphasized the need for ongoing monitoring and regulation at both state and federal levels. National Prescription Drug Take Back Day - October 29, 2022 The Next National Prescription Drug Take Back Day will take place on October 29, 2022 from 10am to 2pm. The National Prescription Drug Take Back Day aims to provide a safe, convenient, and responsible means of disposing of prescription drugs, while also educating the general Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |