Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |
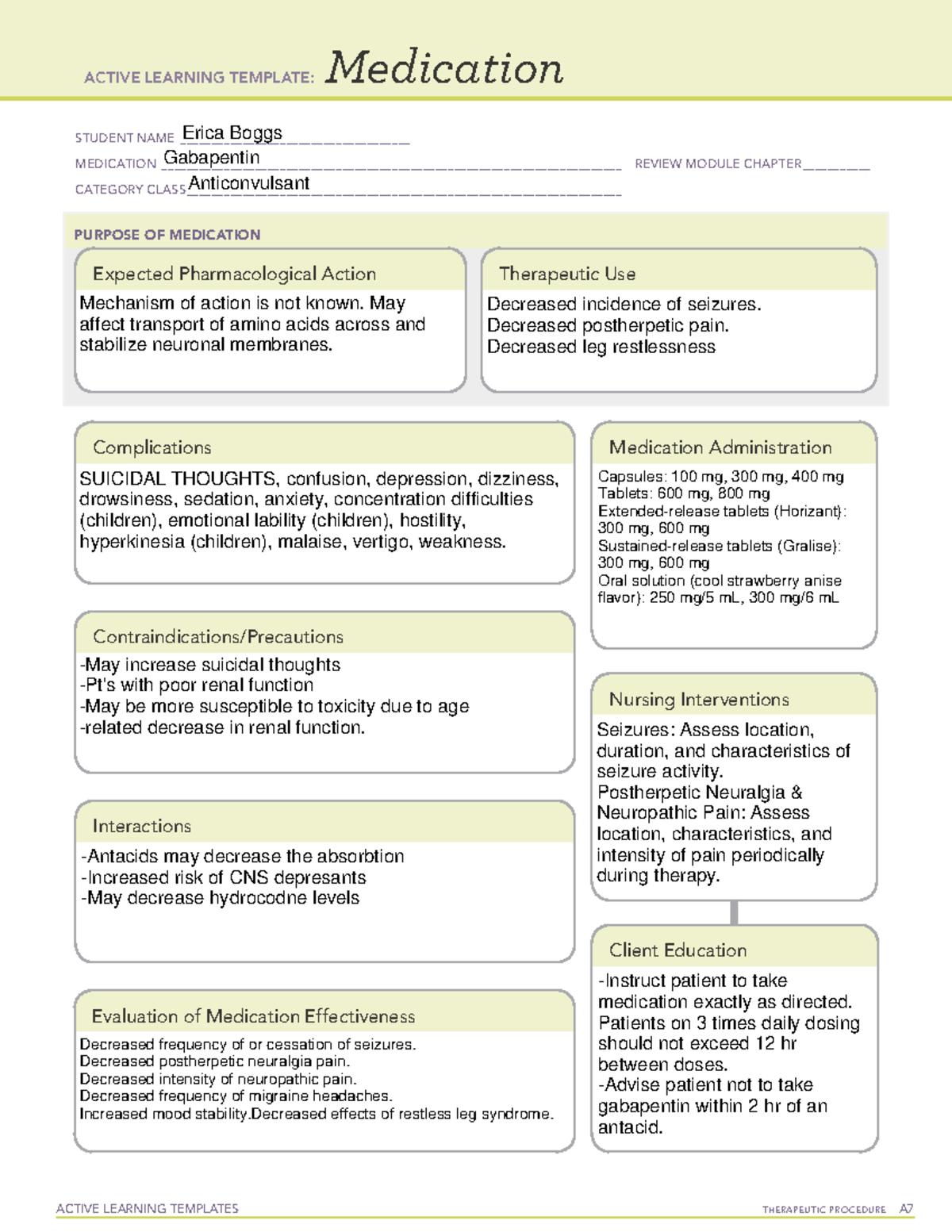
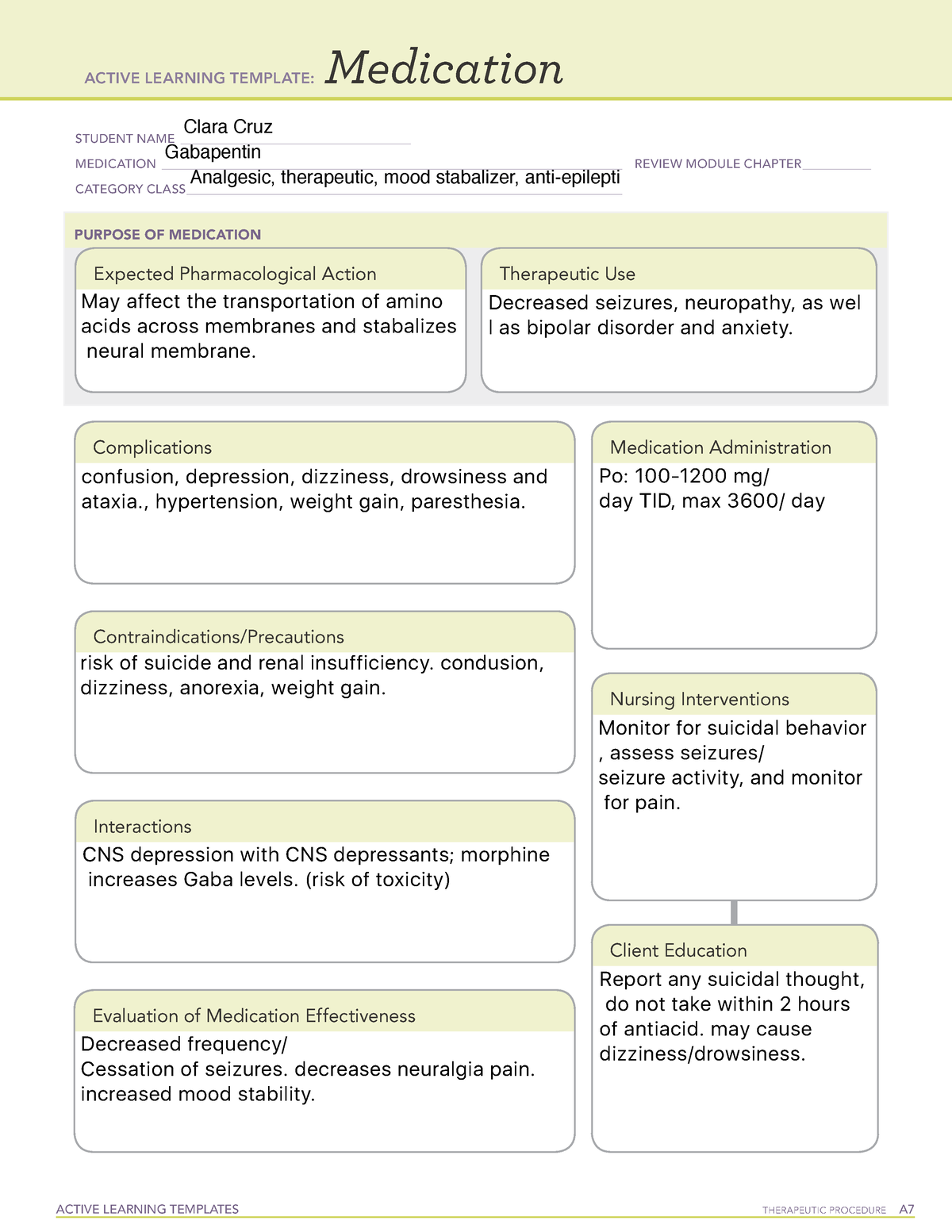
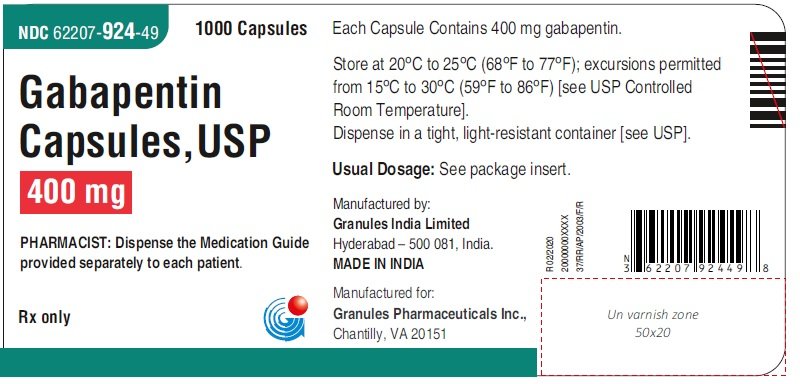
Recent studies raised concerns about the increasing use of gabapentinoids in different countries. With their potential for misuse and addiction, understanding the global consumption of gabapentinoids will offer us a platform to examine the need for any interventional policies. 7 DRUG INTERACTIONS . 7.1 Other Antiepileptic Drugs 7.2 Opioids . 7.3 Maalox ® (aluminum hydroxide, magn esium hydroxide) 7.4 Drug/Laboratory Test Interactions . 8 USE IN SPECIFIC POPULATIONS . 8.1 Pregnancy . 8.2 Lactation 8.4 Pediatric Use . 8.5 Geriatric Use 8.6 Renal Impairment . 9 DRUG ABUSE AND DEPENDENCE . 9.1 Controlled Substance . 9.2 gabapentin has been increasingly encountered by law enforcement, documented in national crime lab reports, reported to poison control centers, and diverted for illicit use. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Taking gabapentin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before taking opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Tell your doctor about all your current medicines. Many drugs can affect gabapentin, especially: naproxen; Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid ([]) that was first approved for use in the United States in 1993.It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures - today it is also widely used to treat neuropathic pain. Drugs Tested: GAB - Gabapentin 2000 ng/ml Description: This single panel drug test dip is a lateral flow chromatographic Immunoassay for the qualitative detection of Gabapentin metabolites in human urine. For the Identify Health drug test package FUO insert please click the icon below: Gabapentin drug usage statistics for the United States (2013 - 2022). Statistics include drug synonyms and therapeutic classes, including: Gabapentin Enacarbil, Gabapentin, Gralise, Neurontin, Horizant, Anti-epileptic Agent. During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for From 2016 through 2020, a total of 333.93 million gabapentin prescriptions and 51.38 million pregabalin prescriptions were dispensed. The highest numbers of prescriptions for both gabapentin and pregabalin were dispensed in 2020. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. Synopsis Gabapentin is an antiepileptic drug with an unknown mechanism of action apparently dissimilar to that of other antiepileptic agents, and possessing some desirable pharmacokinetic traits. The drug is not protein bound, is not metabolised and does not induce liver enzymes, diminishing the likelihood of drug interactions with other antiepileptic agents and drugs such as oral Gabapentin enacarbil is a prodrug of gabapentin. Gabapentin is used in combination with other anticonvulsants for management of partial seizures with or without secondary generalization in adults and children ≥3 years of age. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Gabapentin is available as Gralise, Neurontin, and generic gabapentin in the following dosage forms that are taken by mouth. 100 mg, 300 mg, 400 mg oral capsules 250 mg/5 mL oral solution Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. The novel anticonvulsant drug, gabapentin (neurontin), binds to the subunit of a calcium channel. J Biol Chem 1996; 271: 5768–5776. Crossref. PubMed. Web of Science.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |