Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
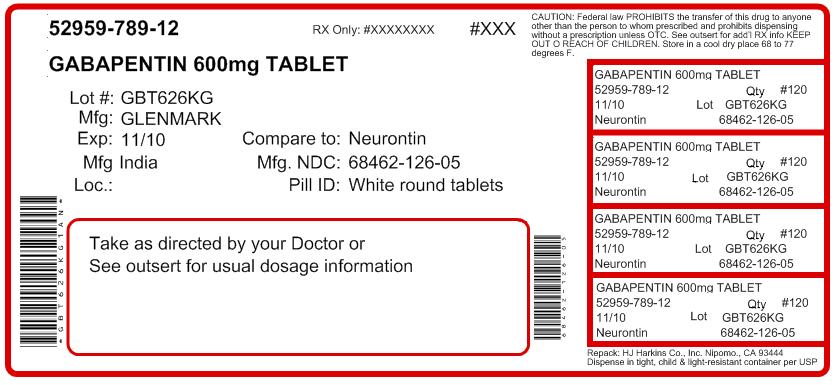
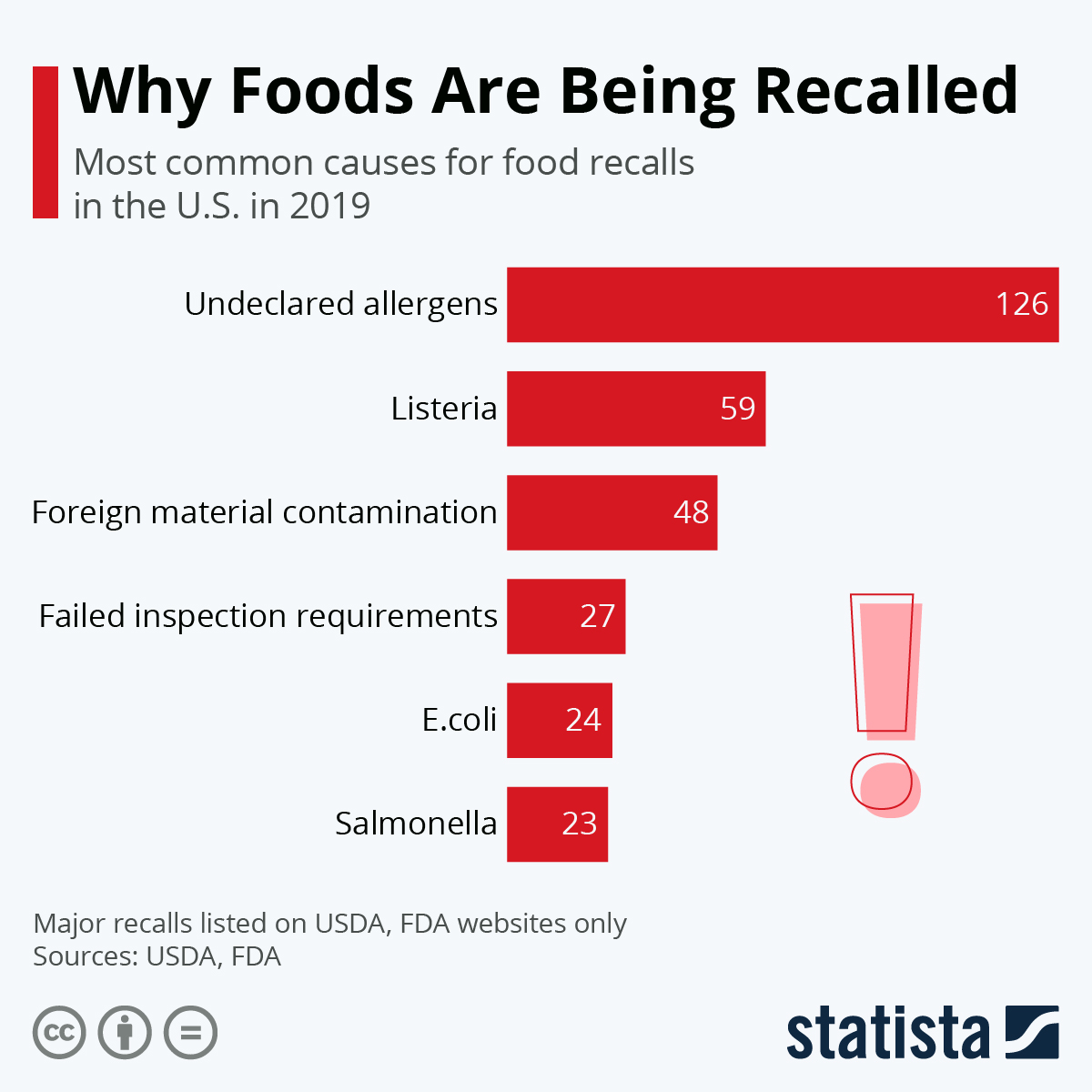
The list below provides information gathered from press releases and other public notices about certain recalls of FDA-regulated products. Not all recalls have press releases or are posted on The last Recall Enforcement Report for Gabapentin with NDC 70010-227 was initiated on 07-31-2024 as a Class II recall and it is currently ongoing. The last Recall Enforcement Report for Gabapentin with NDC 70010-228 was initiated on 07-31-2024 as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets The latest recall number for this product is D-0634-2024 and the recall is currently ongoing . Recall Number Recall Initiation Date Product Description Recall Reason Recall Firm / Quantity Recall Classification Status; D-0248-2025: 02-18-2025: Avastin 1.25 mg/0.05 mL in 0.25 mL Syringe, For Intravitreal Injection Only, Office Use Only - Not for Resale - Single Use, This drug product was repackaged by Turbare Manufacturing, 925 Jeanette Drive, Conway, AR 72032, NDC: 83556-0101-01. Aurobindo Pharma USA, Inc, of Dayton, NJ, has issued a voluntary recall of one lot (Lot Number GESB14011-A) of gabapentin capsules, USP 300 mg, 100-count bottles to the consumer level after finding that some capsules were empty. Get an alert when a recall is issued. Do not use • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. FDA provides a searchable list of recalled products. Drug recalls are actions taken by a firm to remove a product from the market and may be conducted on a firm's own initiative, by FDA Drug Recall Enforcement Report Class III voluntary initiated by The Harvard Drug Group, originally initiated on 04-24-2023 for the product Gabapentin Tablets, USP 600 mg, packaged in Cartons of 100 tablets (10 tablets per blister pack x 10), Rx Only, Distributed by: Aurobindo Pharma USA, Inc. East Windsor, NJ 08520 Distributed by: Major Pharmaceuticals 17177 N Laurel Park Dr., Suite 233 FDA Announces Voluntarily Recall for Batch of Gabapentin 300 MG Capsules Epilepsy News From: Tuesday, November 25, 2014 The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. The recall includes 7,317 devices distributed from May 11, 2018, to Sept. 5, 2019. A class I recall of the Medfusion 4000 Syringe Pump with Firmware Version 1.7.0 by Smiths Medical due to the potential for low-battery alarms to stop working. Drug Recall Enforcement Report Class III voluntary initiated by Sciegen Pharmaceuticals Inc, originally initiated on 02-17-2023 for the product Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05. Gabapentin 100mg Capsule. On April 22, 2020, Aurobindo Pharma USA, Inc., recalled GABAPENTIN 100mg due to the possibility of contamination. The U.S. Food and Drug Administration (FDA) has issued a Class II recall of the affected medications. More information about the recall is at: The last Recall Enforcement Report for Gabapentin with NDC 0904-6823 was initiated on 04-24-2023 as a Class III recall due to product mixup: one foreign tablet found in product. The latest recall number for this product is D-0570-2023 and the recall is currently terminated as of 04-30-2024 . Drug Recall Enforcement Report Class II voluntary initiated by Granules Pharmaceuticals Inc., originally initiated on 07-31-2024 for the product Gabapentin Tablets, USP, 600 mg, 500-count bottles, Rx only, Manufactured by: Granules India Limited Hyderabad-500 081, India, Manufactured for: Granules Pharmaceuticals Inc., Chantilly, VA NDC 70010 Gabapentin is being recalled due to a potential issue with empty capsules within certain batches. This isn’t a widespread recall of all gabapentin products, but rather a specific batch of 300 mg capsules manufactured by Aurobindo Pharma USA. The Harvard Drug Group is pulling 3984 cartons of gabapentin tablets after a foreign tablet was found in a carton, according to the May 17, 2023, US Food and Drug Administration (FDA) Enforcement Report. GRANULES PHARMACEUTICALS INC. is recalling Gabapentin Tablets USP, 600mg. This recall is due to a potential mix-up of Metformin ER Tablets in a bottle of Gabapentin Tablets, USP 600mg. Pregabalin (Lyrica) and gabapentin (Neurontin) are both gabapentinoids, a class of nerve medication initially developed to treat epileptic seizures. Sales of Lyrica and Neurontin tripled a decade ago, when they were touted as safer alternatives to opioids and prescribed off-label for a variety of pain conditions. Date of Recall/Manufacturer Drug/Device Name; 3/28/25 Breckenridge Pharmaceutical, Inc. Duloxetine Delayed-Release Capsules, USP, 30mg: Gabapentin Tablets, 600 mg: Class 1 Recall: Reasonable probability that using the drug will cause serious adverse health consequences or death. Class 2 Recall: Using the drug may cause temporary or medical reversible adverse health consequences, the probability of serious adverse health consequences is remote.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |