Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
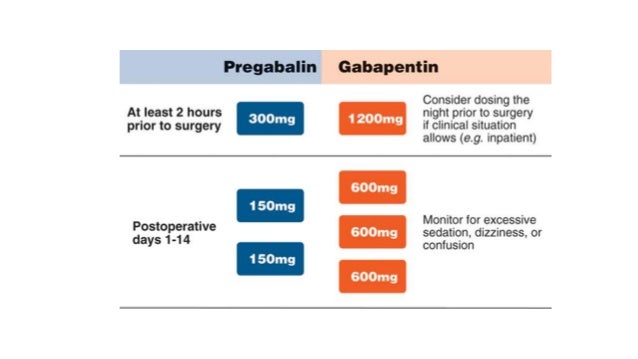
Gabapentin (GBP) has been shown to be effective an add-on drug for the treatment of refractory partial epilepsy. We undertook an open clinical trial to test its efficacy for the first Gabapentin is a structural analogue of GABA used in the treatment of the partial epilepsies of adult and child of more than 12 years, in monotherapy or in association with other anticonvulsant drugs. In association, gabapentin presents the advantage of not interfering with the other anticonvulsant d Therapeutic ranges are based on specimens collected immediately before the next dose (ie, trough). Most epileptic patients show a response to the drug when the trough concentration is in the range of 2 to 20 mcg/mL. Therapeutic drug monitoring may be useful due to inter-individual variation in pharmacokinetics and dose-dependent bioavailability; specimens for measurements should be collected The aim of the study was to investigate the use and pharmacokinetic variability of gabapentin in epilepsy and non-epilepsy indications and to further evaluate the use of TDM in patients with restless legs syndrome (RLS). Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Therapeutic drug monitoring may be useful due to inter-individual variation in pharmacokinetics and dose-dependent bioavailability; specimens for measurements should be collected before the morning dose since the short half-life may affect the interpretation of the concentration. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. A prescription drug monitoring program (PDMP) is an electronic database that tracks controlled substance prescriptions. Information from PDMPs can help clinicians identify patients who may be at risk for overdose and provide potentially lifesaving information and interventions. This study aimed to evaluate gabapentin (GAB) trough plasma concentration range and the applicability of therapeutic drug monitoring in patients with neuropathic pain. Fifty-three patients with neuropathic pain, aged 20 to 75, received gabapentin as treatment for at least 7 days. Therapeutic drug monitoring may be useful due to inter-individual variation in pharmacokinetics and dose-dependent bioavailability; specimens for measurements should be collected before the morning dose since the short half-life may affect the interpretation of the concentration. This activity outlines the indications, mechanisms of action, administration, significant adverse effects, contraindications, monitoring, and characteristics of gabapentin toxicity. Oregon's Prescription Drug Monitoring Program (PDMP) is a program developed to promote public health and welfare and help improve patient care. The information will aid healthcare providers and pharmacists to better manage patients' prescriptions to improve quality of care. It will also support the appropriate use of prescription drugs. While gabapentin is generally considered safe, pharmacists play a crucial role in assisting the healthcare team and monitoring patients' medication regimens to prevent potential drug interactions. In a documented case, a patient with diabetes, diabetic neuropathy, and hypertension was prescribed both gabapentin and pregabalin, resulting in Ikeda K, Ikawa K, Yokoshige S, Yoshikawa S, Morikawa N. Gas chromatography-electron ionization-mass spectrometry quantitation of valproic acid and gabapentin, using dried plasma spots, for therapeutic drug monitoring in in-home medical care. Gabapentin has been increasingly used in various indications in recent years. Despite variable pharmacokinetics, therapeutic drug monitoring (TDM) is scarcely described in other indications than epilepsy. Therapeutic drug monitoring has lower utility for gabapentin, pregabalin, and vigabatrin. Measurement of salivary drug concentrations has potential utility for therapeutic drug monitoring of lamotrigine, levetiracetam, and topiramate. Gabapentin is cleared by the kidneys and has an elimination half-life of five to seven hours. 1 Steady-state concentrations are reached after one to two days with the time between ingestion and maximal serum concentration being two to three hours. 2 For therapeutic drug monitoring, specimens should be drawn as trough levels. There are no Gabapentin is frequently combined with other substances for the purpose of potentiating the effects of the drugs or achieving a “high.” Studies have identified various substances that are commonly abused in combination with gabapentin, including alcohol, opioids, benzodiazepines, antidepressants, and other CNS depressants 13,14,15. The Oregon Prescription Drug Monitoring Program (PDMP) is a tool to help healthcare providers and pharmacists provide patients better care in managing their prescriptions. It contains information provided by Oregon-licensed retail pharmacies.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |