Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
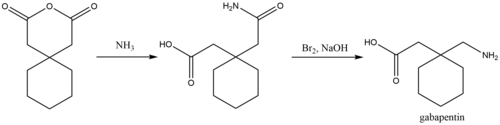 |  |
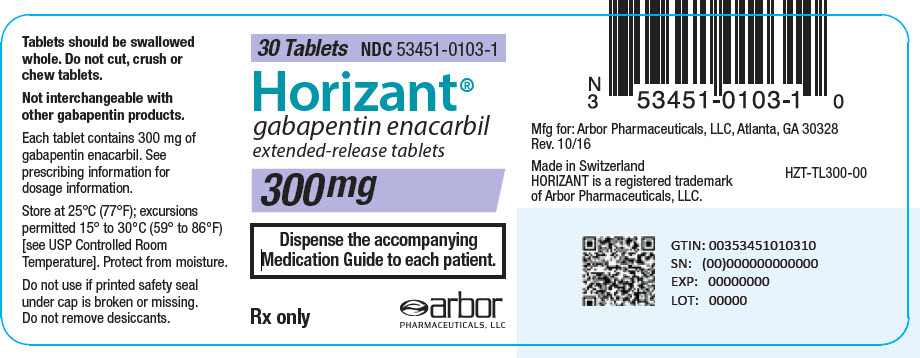
17.5 Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity . 17.6 Lack of Interchangeability With Gabapentin 17.7 Dosing Instructions . 17.8 Alcohol * Sections or subsections omitted from the full prescribing information are not listed. 1. Reference ID: 4584082 . This label may not be the latest approved by FDA. (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients The US Food and Drug Administration (FDA) has approved gabapentin enacarbil (Horizant) extended-release tablets for the management of postherpetic neuralgia in adults. The drug, approved in April 2011 for the treatment of restless legs syndrome (RLS), is now approved to treat PHN at a dose of 600 mg twice daily, notes a joint statement from HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use Initial U.S. Approval: 2011 -----RECENT MAJOR CHANGES-----Indications and Usage, Management of Postherpetic Neuralgia (1.2) 06/2012 Dosage and Administration, Postherpetic Neuralgia (2.2) 06/2012 Horizant is a brand name of gabapentin enacarbil, approved by the FDA in the following formulation (s): Has a generic version of Horizant been approved? No. There is currently no therapeutically equivalent version of Horizant available in the United States. Gabapentin enacarbil was denied approval by the U.S. Food and Drug Administration (FDA) in February 2010, citing concerns about possible increased cancer risk shown by some animal studies. Please refer to your supplemental new drug application (sNDA) dated and received January 17, 2020, submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (FDCA) for Horizant (gabapentin enacarbil). We also refer to our letter dated December 19, 2019, notifying you, under Section Post-Approval Agreements: None (b) Drug Substance: Gabapentin enacarbil (also called XP 13512) is a non-ester, pro-drug of gabapentin (a marketed drug). It has a molecular formula C16H27NO6 and molecular weight 329.39. There is a single chiral center , the drug substance is a racemic mixture. Gabapentin enacarbil is a white to off-white powder, GlaxoSmithKline plc (GSK) and XenoPort, Inc. (Nasdaq: XNPT) announced today that the United States (US) Food and Drug Administration (FDA) has approved Horizant; (gabapentin enacarbil) Extended-Release Tablets for the management of postherpetic neuralgia (PHN) in adults. concentrations of gabapentin relative to other gabapentin products. [See Clinical Pharmacology : 76 (12.3).] 77 : 78 ; The safety and effectiveness of HORIZANT in patients with epilepsy have not been studied. 79 ; 80 : 5.4 Suicidal Behavior and Ideation : 81 : HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin, an antiepileptic drug Gabapentin enacarbil is a prodrug of gabapentin, an antiepileptic drug (AED). AEDs increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Horizant (gabapentin enacarbil) Extended-Release Tablets Company: GlaxoSmithKline Application No.: 022399 Approval Date: 4/06/2011. Persons with disabilities having problems accessing Horizant (gabapentin enacarbil) is used to treat restless legs syndrome and nerve pain caused by the herpes virus. Includes Horizant side effects, interactions and indications. Gabapentin enacarbil is a prodrug of gabapentin and, accordingly, its therapeutic effects in restless legs syndrome and postherpetic neuralgia are attributable to gabapentin. In animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to Section 505(o)(3) of the FDCA authorizes FDA to require holders of approved drug and biological product applications to conduct postmarketing studies and clinical trials for certain purposes, if FDA makes certain findings required by the statute. We have determined that an analysis of spontaneous postmarketing adverse events reported Horizant FDA Approval History. FDA Approved: Yes (First approved April 6, 2011) Brand name: Horizant Generic name: gabapentin enacarbil Dosage form: Extended Release Tablets Previous Name: Solzira Company: Azurity Pharmaceuticals, Inc. Treatment for: Restless Legs Syndrome, Postherpetic Neuralgia Issued: London, UK, Research Triangle Park, NC & Santa Clara, CA. New treatment for moderate-to-severe primary Restless Legs Syndrome; GlaxoSmithKline (NYSE: GSK) and XenoPort, Inc. (Nasdaq: XNPT) announced Wednesday that the U.S. Food and Drug Administration (FDA) has approved Horizant™ (gabapentin enacarbil) Extended-Release Tablets for the treatment of moderate-to-severe primary Restless April 11, 2011 (UDDATED April 28, 2011) — The US Food and Drug Administration (FDA) has approved gabapentin enacarbil extended-release tablets (Horizant; GlaxoSmithKline and XenoPort Inc) for Horizant® is the only FDA-approved alpha-2-delta ligand for treatment of moderate-to-severe Restless Legs (RLS) in adults 1,2. Learn more about how Horizant® relieved the symptoms of RLS patients in a clinical trial gabapentin enacarbil Trade Name: Horizant Marketing Approval Date: 06/06/2012 Approved Labeled Indication: Management of postherpetic neuralgia in adults. Exclusivity End Date: 06/06/2019
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |