Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
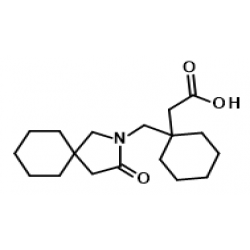 |  |
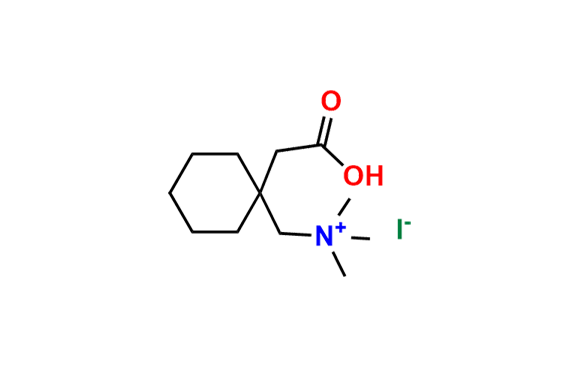
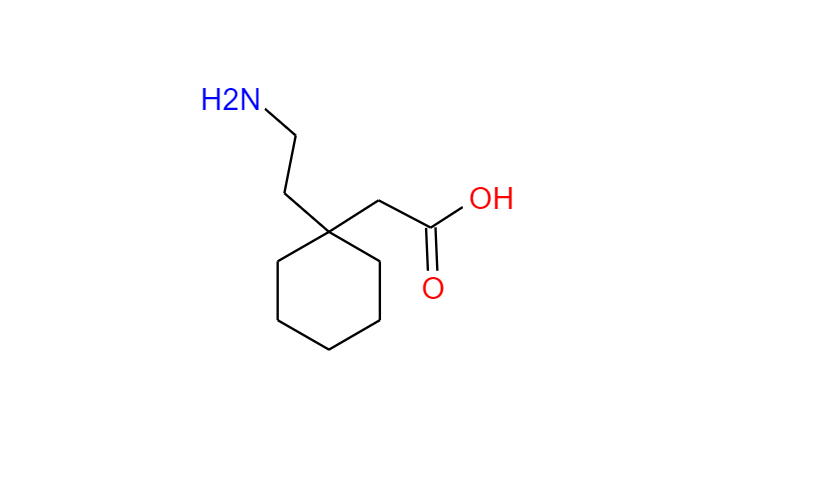
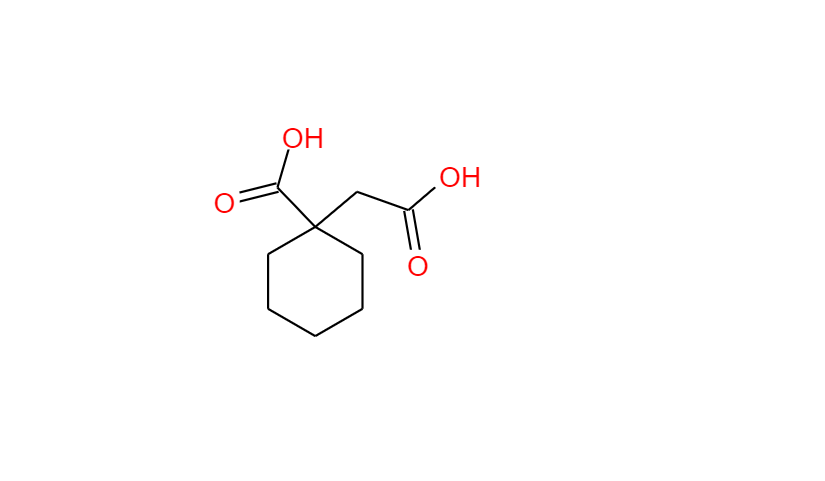
GABAPENTIN Product Monograph Page 3 of 32 Pr GABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg, and 400 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Non-medicinal Ingredients Oral Capsules / 100 mg, 300 mg, and 400 mg Corn Starch, Lactose Anhydrous, and Talc. Buy high-quality Gabapentin EP Impurity A from SynZeal. Gabapentin EP Impurity A Reference Standard is supplied with COA and analytical data. CAS No. 64744-50-9, Synonyms: Gabapentin USP Related Compound A ; Gabapentin Lactam ;, Chemical name: 2-Azaspiro-[4,5]decan-3-one ; 3,3-Pentamethylene-5-butyrolactam ; 4,4-Pentamethylene-2-pyrrolidinone ;. Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (2024). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. in which C S is the concentration, in mg per mL, of USP Gabapentin Related Compound A RS in the Standard solution; C T is the concentration, in mg per mL, of gabapentin in the Test solution, based on the label claim; and r U and r S are the individual peak responses for gabapentin related compound A obtained from the Test solution and Standard Neurontin (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). Microsoft Word - Gabapentin Capsules_BP2017 Author: wallm Created Date: 8/24/2016 10:03:38 AM GABAPENTIN Product Monograph Page 3 of 32 PrGABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Non-medicinal Ingredients Oral Capsules: 100 mg, 300 mg, and 400 mg Corn Starch, Lactose Anhydrous, and Talc. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a tive to gabapentin related compound D) which is 1.0 for USP Gabapentin RS gabapentin related compound D and 0.025 for all other impuri-USP Gabapentin Related Compound A RS ties, respectively; C 2-Aza-spiro[4.5]decan-3-one. S is the concentration, in mg per mL, of USP Gabapentin Related Compound D RS in the Standard solution; C9H15NO 153.22 C TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. » Gabapentin Tablets contain not less than 90.0 percent age; and L is the Tablet label claim, in mg. and not more than 110.0 percent of the labeled amountTolerances—Not less than 80% (Q) of the labeled amount of of gabapentin (C 9H 17NO 2). gabapentin (C 9H 17NO 2) is dissolved in 45 minutes. Gabapentin Product Monograph Page 1 of 32 PRODUCT MONOGRAPH PrGabapentin Capsules USP PrGabapentin Tablets USP Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Accord Healthcare Inc. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). Gabapentin (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Geriatrics (>65 years of age) Buy Gabapentin Ph Eur reference standard for identification, purity tests or assays of pharmaceutical products according to EP monographs. 6- Used in monograph(s) lists the European pharmacopoeia monograph(s) and/or general method(s) prescribing the use. For more details, see leaflet. 7- Assigned content See leaflet for the value and explanations on use. Guidance Document for Drafting and Formatting of Monographs for Indian Pharmacopoeia; IP Review Process; Stakeholder Comments. New & Revised General Chapter / Monographs - For Comments; Amendments Proposed to IP 2022 - For Comment; Monographs Inclusion-Exclusion Criteria; SOP for Development of IP Monograph ; Meeting of Expert Working Groups Gabapentin enacarbil is used for symptomatic treatment of moderate-to-severe primary restless legs syndrome (Ekbom syndrome) in adults. Not recommended in patients who are required to sleep during the daytime and remain awake at night. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of This chromato ogram is provided for information only as an aid to analy ysts and intended as guidance for the interpretation and application of BP monographs. Typical chrom matogram fo or solution (4) at 210 nm in the Related Substtances test for Gabapentin Tablets as published in BP 2017.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |