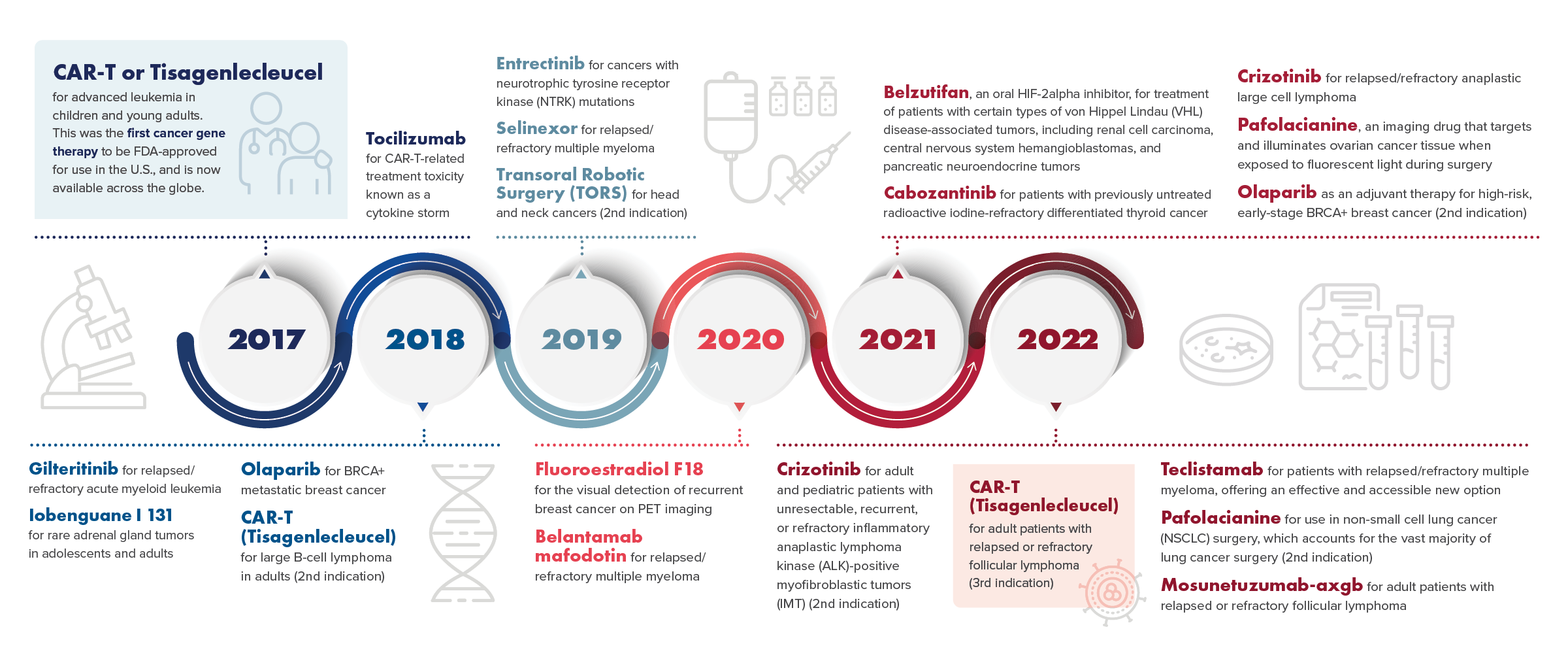
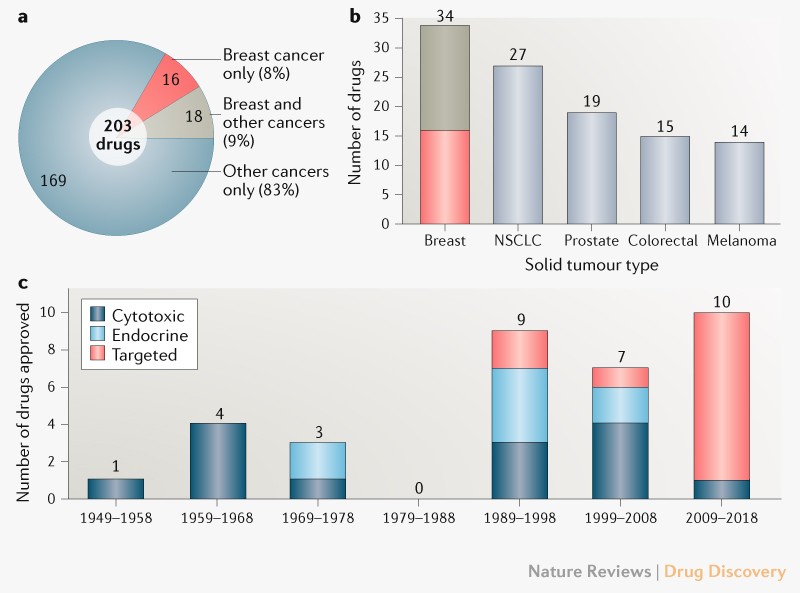
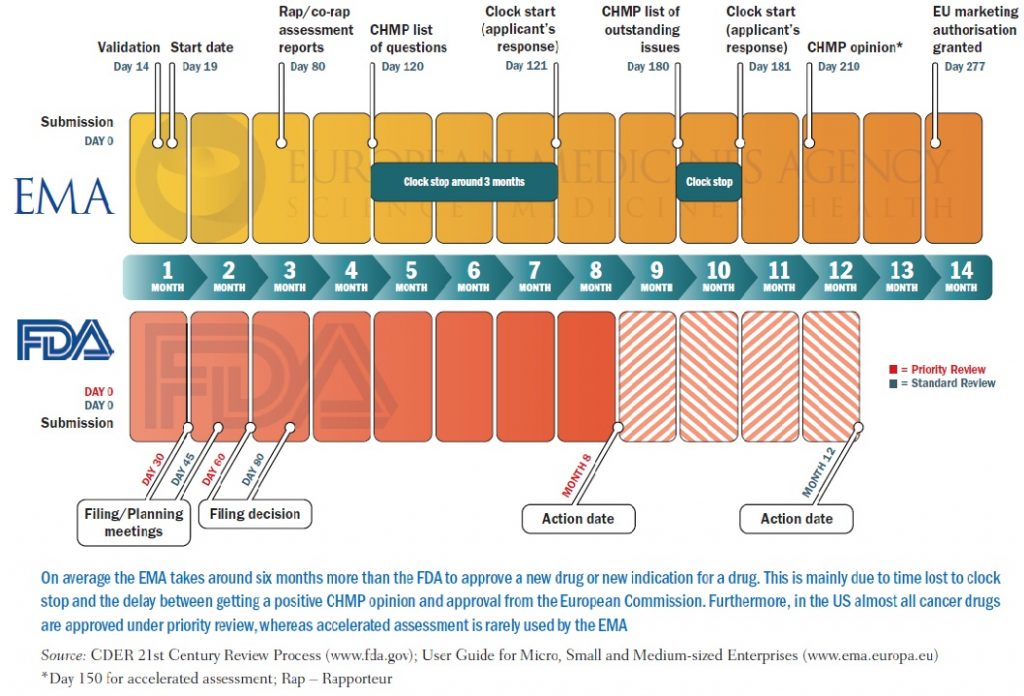
Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
Gabapentin (Neurontin, Gralise, Horizant) was first approved by the US Food and Drug Administration (FDA) in 1993 for the treatment of epilepsy, and in 2004 was approved for the treatment of post-herpetic neurological pain. 1 However, almost 95% of gabapentin prescriptions are for off-label use, including for some forms of anxiety disorders The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Neurontin (Gabapentin) Oral Solution, Capsules & Tablets Company: Parke-Davis Application No.: 021216/020235S015/020882S002/021129S005 Approval Date: 10/12/2000. Approval Letter(s) (PDF) Gralise FDA Approval History. FDA Approved: Yes (First approved January 28, 2011) Brand name: Gralise Generic name: gabapentin Dosage form: Extended Release Tablets Previous Name: DM-1796 Company: Depomed, Inc. Treatment for: Postherpetic Neuralgia Horizant FDA Approval History. FDA Approved: Yes (First approved April 6, 2011) Brand name: Horizant Generic name: gabapentin enacarbil Dosage form: Extended Release Tablets Previous Name: Solzira Company: Azurity Pharmaceuticals, Inc. Treatment for: Restless Legs Syndrome, Postherpetic Neuralgia Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. Gabapentin gained FDA approval in 1993 under the brand name Neurontin as an adjunctive therapy in the treatment of partial onset seizures, and subsequently for the treatment of postherpetic neuralgia in adults in 2002. It became available as a generic in 2004. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. History. In 1993, the FDA approval of Neurontin, the original branded gabapentin, was for use as an adjunctive medication to control partial seizures. 9 Over the next several years, the manufacturer, Parke-Davis, a subsidiary of Warner-Lambert, engaged in a large marketing campaign to increase off-label prescribing of Neurontin for pain. 4 By 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or FDA approved labeling text (dated 10/12/00) Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. One such drug, gabapentin (Neurontin), received approval by the U.S. Food and Drug Administration (FDA) in 1993 as an adjunct medicine for partial seizures and additional FDA approval in 2002 for Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in Gabapentinoid products include gabapentin, which is marketed under the name Neurontin Gralise (gabapentin) Tablets 300 mg and 600 mg Company: Abbott Products, Inc. Application No.: 022544 Approval Date: 01/28/2011. Persons with disabilities having problems accessing the PDF files below may call (301) 796-3634 for assistance.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |