Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
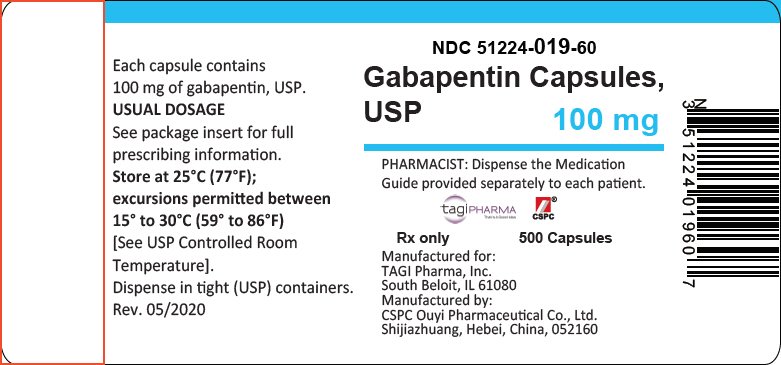 |  |
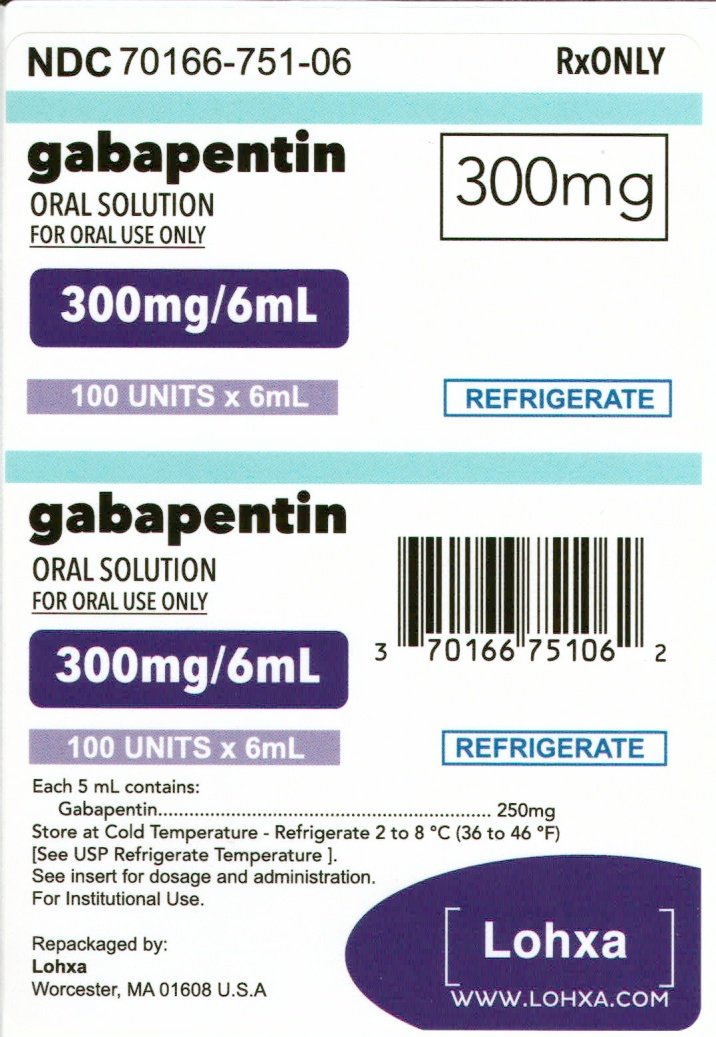
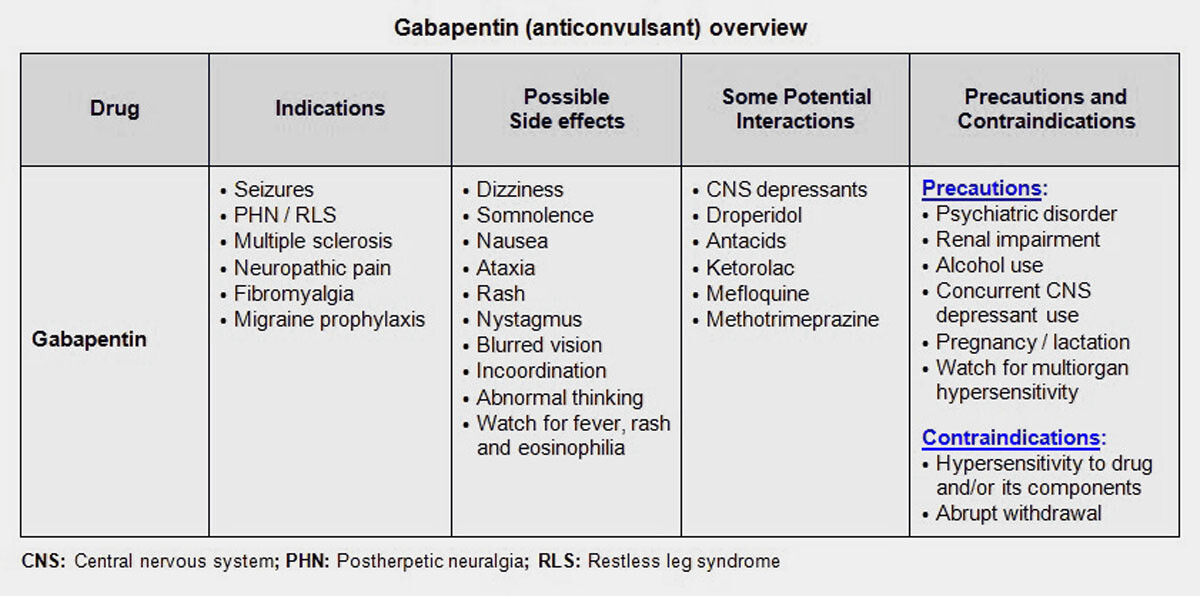
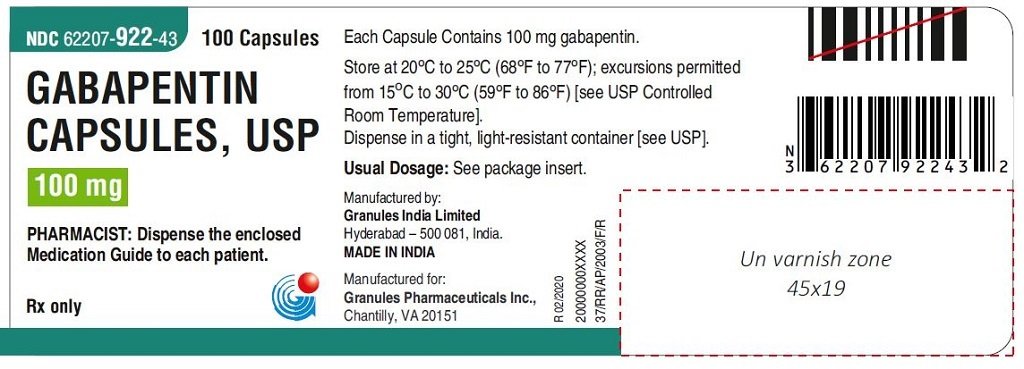
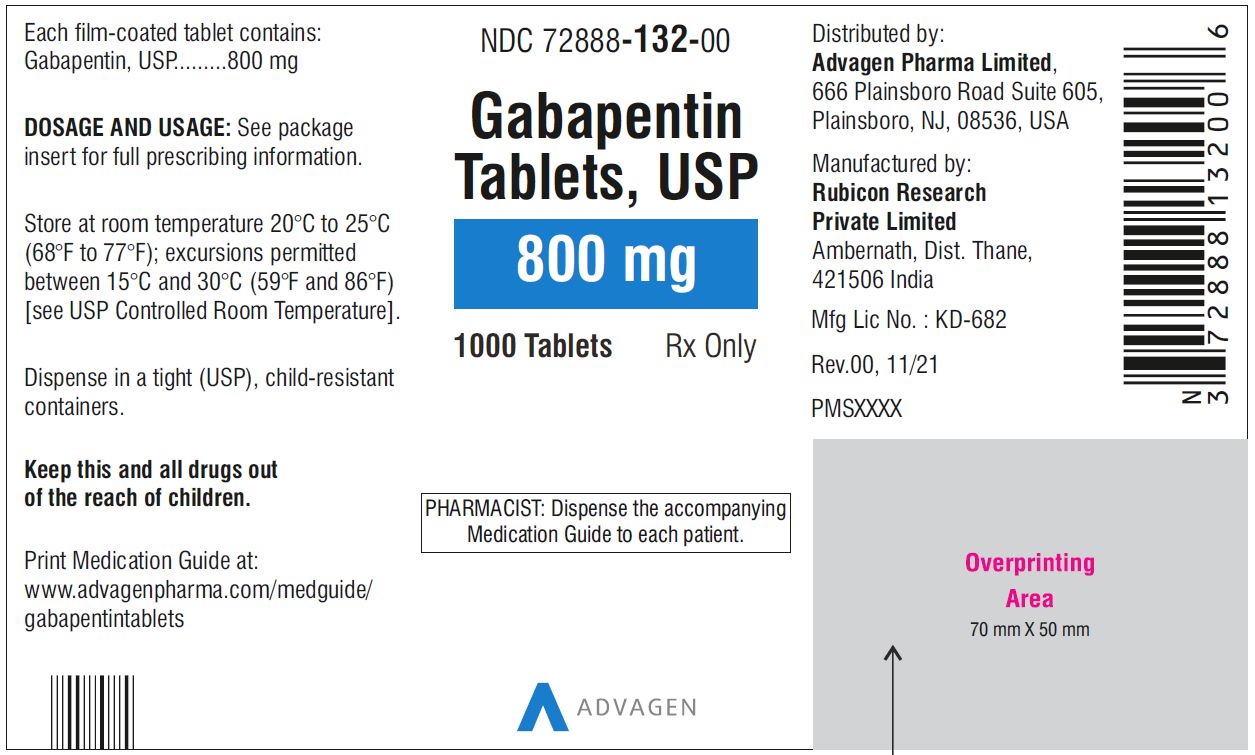
gabapentin has been increasingly encountered by law enforcement, documented in national crime lab reports, reported to poison control centers, and diverted for illicit use. Licit Uses: According to the FDA-approved product label, gabapentin is used clinically for the management of postherpetic neuralgia in adults and as This label may not be the latest approved by FDA. For current labeling information, please visit 11-point numeric pain rating scale ranging from 0 (no pain) to hypersensitivity to the drug HIGHLIGHTS OF PRESCRIBING INFORMATION . These highlights do not include all the information needed to use GRALISE safely and effectively. See full prescribing information for GRALISE. GRALISE™ (gabapentin) tablets Initial U.S. Approval: 1993 -----INDICATIONS AND USAGE ----- FDA-Approved Indications. Gabapentin: Gabapentin is indicated for postherpetic neuralgia and serves as adjunctive therapy for managing partial seizures (with or without secondary generalization) in adults and pediatric patients aged 3 or older. NDA 022399 – FDA Approved Labeling Text dated December 2012 . HIGHLIGHTS OF PRESCRIBING INFORMATION . These highlights do not include all the information needed to use HORIZANT safely and effectively. See full prescribing information for HORIZANT. HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use Initial U.S. Approval: 2011 Gabapentin is FDA approved for pain management of a limited number of neuropathic pain conditions Gabapentin is widely used off-label for various chronic pain conditions and for the treatment of acute pain, making it now one of the most commonly described analgesic drugs Table 1: FDA-Approved Indications for Pregabalin and Gabapentin: Indications. Pregabalin. Gabapentin. Neuropathic pain associated with diabetic peripheral neuropathy. x. Different brands of gabapentin are not interchangeable and they are FDA approved for different conditions. Use only the brand and form of gabapentin your doctor has prescribed. Check your medicine each time you get a refill to make sure you receive the correct form. FDA Approved Labeling Text dated 03/01/2011 Page 2 . particular, gabapentin prevents pain-related responses in several models of neuropathic pain in rats or mice (e.g. spinal nerve ligation Page 2 of 24 1 FULL PRESCRIBING INFORMATION 2 . GRA-004-C.5 SEP 2012 3 GRALISE® (gabapentin) Tablets 4 . 1 INDICATIONS AND USAGE 5 . GRALISE is indicated for the management of postherpetic neuralgia. ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors 17.5 Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity . 17.6 Lack of Interchangeability With Gabapentin 17.7 Dosing Instructions . 17.8 Alcohol * Sections or subsections omitted from the full prescribing information are not listed. 1. Reference ID: 4584082 . This label may not be the latest approved by FDA. Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times ----- INDICATIONS AND USAGE-----gabapentin products because of differing pharmacokinetic profil with the evening meal. GRALISE alternative medication, this should be done gradually over a mi function. GRALISE should not be us hypersensitivity to the drug ----- RECENT MAJOR CHANGES----- 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. Owing to the multiple actions of the GABA system, gabapentin has subsequently been used for a wide variety of conditions, with up to 95% of gabapentin today prescribed for off-label indications. 2,3 Prescribers are often unaware of gabapentin’s approved indications and their prescribing of gabapentin is largely guided by informal discussion Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |