Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
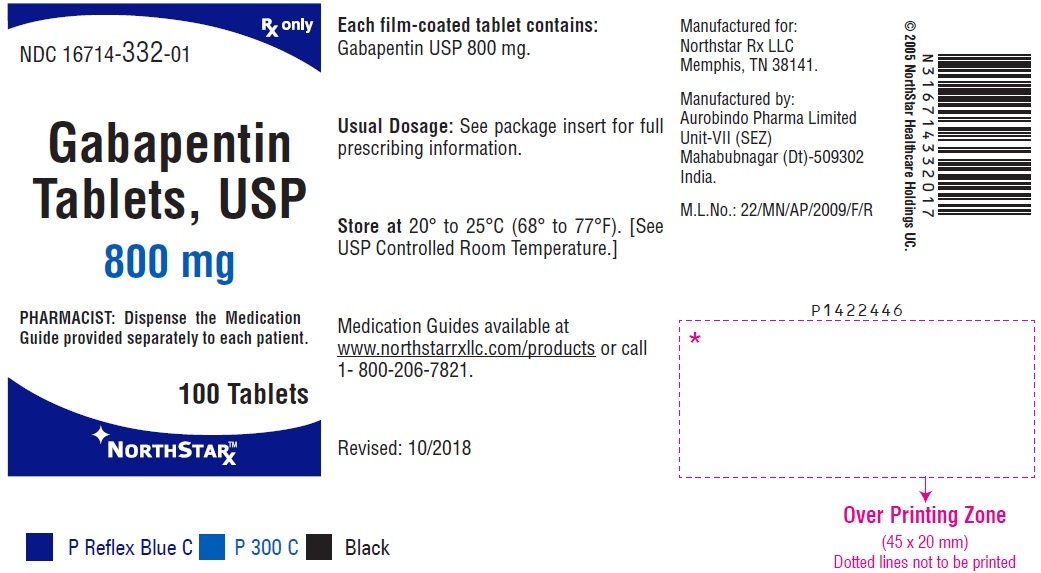 |  |
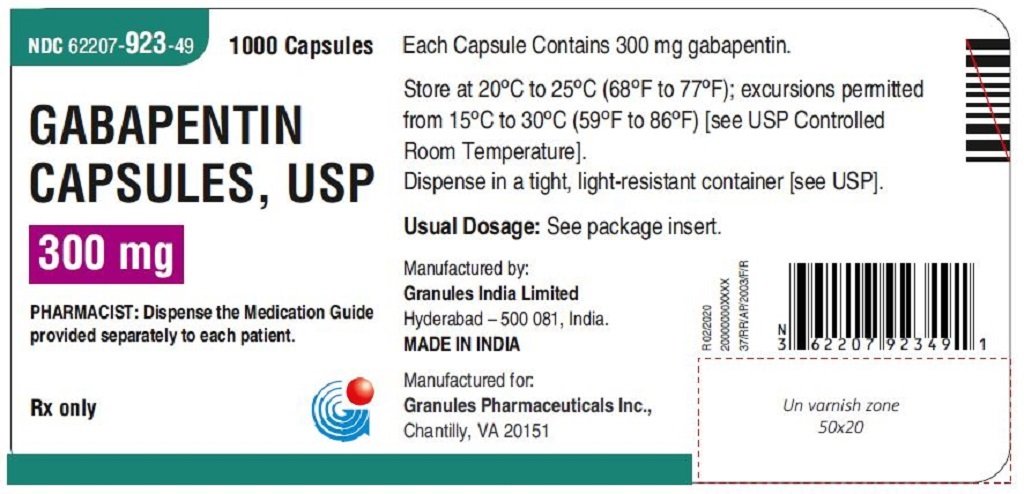
FDA warns that geriatric patients receiving gabapentin are at increased risk of potentially serious, life-threatening, or fatal respiratory depression; initiate therapy with the lowest dosage and titrate carefully with close monitoring. There was a larger treatment effect in patients 75 years of age and older compared to younger patients who received the same dosage. Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients ≥ 75 years may be a consequence of increased gabapentin exposure for a given dose that Usual Adult Dose for Restless Legs Syndrome: Gabapentin enacarbil available under the trade name Horizant: 600 mg orally once daily with food at about 5 PM Use: For the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. Usual Pediatric Dose for Epilepsy: Less than 3 years: Not recommended A dose of 1,200 mg once daily provided no additional benefit compared . with the 600-mg dose, but caused an increase in adverse reactions. (2.1) If the dose is not taken at the recommended time, the next dose should be . taken the following day as prescribed. (2.1) PHN: The starting dose is 600 mg in the morning for 3 days, then increase to Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly • A dose of 1,200 mg once daily provided no additional benefit compared with the 600-mg dose, but caused an increase in adverse reactions. (2.1) • If the dose is not taken at the recommended time, the next dose should be taken the following day as prescribed. (2.1) PHN: The starting dose is 600 mg in the morning for 3 days, then increase to Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Apply patient-centered approaches to gabapentin prescribing, tailoring dosage adjustments and treatment plans based on individual needs and preferences. Detailed Gabapentin dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments. The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in three divided doses, and the recommended maintenance dose reached by upward titration over a period of Gabapentin should be administered three times a day using 300 mg or 400 mg capsules. The maximum time between doses should not exceed 12 hours. gabapentin products because of differing pharmacokinetic profil with the evening meal. GRALISE alternative medication, this should be done gradually over a mi function. GRALISE should not be us hypersensitivity to the drug ----- RECENT MAJOR CHANGES----- ----- DOSAGE AND ADMINISTRATION ----- Administer gabapentin three times a day using 300 mg or 400 mg capsules. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 Years. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are Administer gabapentin three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 years. Concurrent drug therapy issues: Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information. Dosage form specific issues: There was a larger treatment effect in patients 75 years of age and older compared to younger patients who received the same dosage. Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients ≥ 75 years may be a consequence of increased gabapentin exposure for a given dose that The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in three divided doses, and the recommended maintenance dose reached by upward titration over a period of approximately An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate releaseby about Gabapentin is FDA approved for pain management of a limited number of neuropathic pain conditions Gabapentin is widely used off-label for various chronic pain conditions and for the treatment of acute pain, making it now one of the most commonly described analgesic drugs
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |