Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
+and+Gabapentin+(Neurontin).jpg) |  |
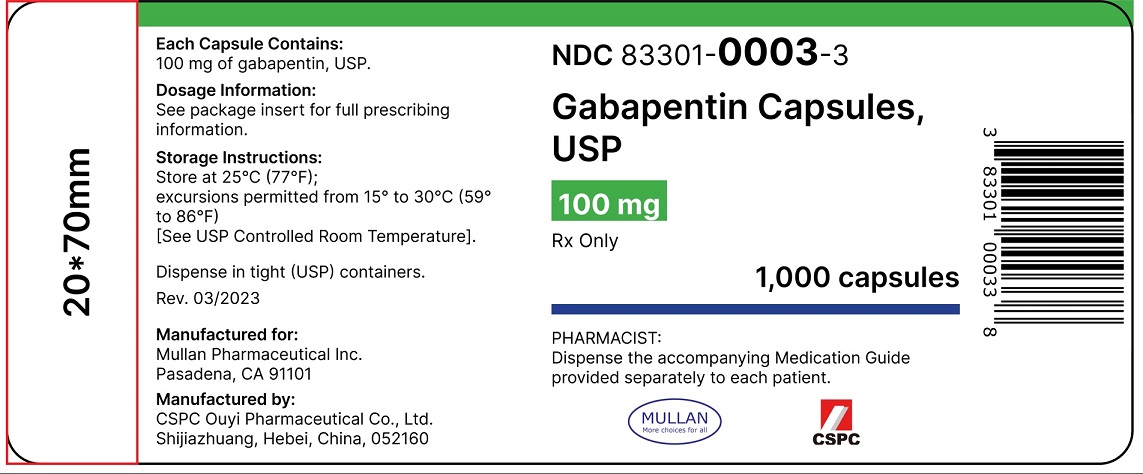
Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. **The effective date of the FDA guidance for industry, Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) is August 7, 2023 and the effective date of During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. 1 Links in the flush list direct you to specific disposal instructions in each medicine’s labeling (the labeling generally represents the last FDA-approved labeling at the time this webpage was Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly In contrast, new evidence supporting three alpha-2-delta ligand calcium channel blockers — gabapentin enacarbil, gabapentin, and pregabalin — led the task force to support them as strong recommendations for RLS treatment. These medications are not associated with the augmentation of RLS symptoms observed with the dopaminergic agents. Waiver request of in-vivo testing: 100mg, 300mg, 400mg, and 600 mg based on (i) acceptable bioequivalence studies on the 800 mg strength, (ii) proportionally similar across all strengths, and (iii) Active Ingredient: Gabapentin . Dosage Form: Tablet . Route: Oral . Strengths: 300 mg, 450 mg, 600 mg, 750 mg, 900 mg. Recommended Studies: Three in vivo bioequivalence studies with FDA is warning that serious, life-threatening, and fatal respiratory depression has been reported with the gabapentinoids, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in patients who use gabapentanoids with opioid pain medicines or other drugs that depress the Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Guidelines by several professional societies (ASA, ASRA, APS) advocate for perioperative administration of gabapentin as a component of multimodal analgesia. However, recent publications challenge the routine use of gabapentin in the perioperative setting due to a potentially unfavorable ratio of benefit vs. adverse side effects. Gabapentin is currently FDA-approved for use in postherpetic neuralgia and particular seizure disorders2. However, it is frequently used for the following off-label uses 3, 4, 5: Sleep disorders. Sciatic back pain. Diabetic neuropathy. Fibromyalgia. Migraine prophylaxis. Restless leg syndrome. Perimenopausal hot flashes. Mood disorders. PTSD. 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Detailed Gabapentin dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
+and+Gabapentin+(Neurontin).jpg) |  |