Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
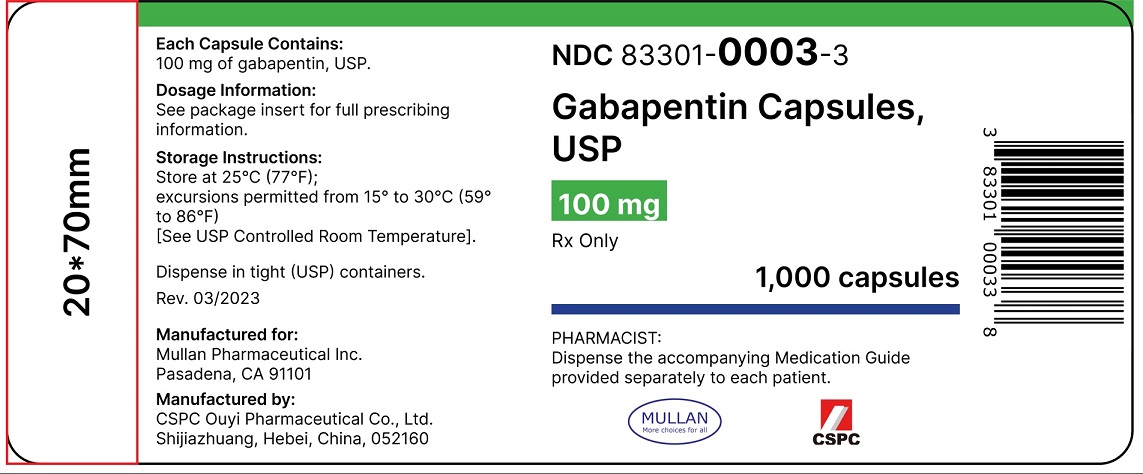
5.3 Withdrawal of Gabapentin 5.4 . Tumorigenic Potential 5.5 . Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity 5.6. Laboratory Tests 6 ADVERSE REACTIONS . 6.1 Clinical Trials Experience . 6.2 Postmarketing and Other Experience with other Formulations of Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate releaseby about Some of these individuals were taking higher than recommended doses of gabapentin for unapproved uses. When prescribing gabapentin, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). 17.5 Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity . 17.6 Lack of Interchangeability With Gabapentin 17.7 Dosing Instructions . 17.8 Alcohol * Sections or subsections omitted from the full prescribing information are not listed. 1. Reference ID: 4584082 . This label may not be the latest approved by FDA. 6.2 Adverse Events Associated With Gabapentin 17.3 Suicidal Behavior and Ideation 7 DRUG INTERACTIONS 17.4 Drug Reaction With Eosinophilia and Systemic 8 USE IN SPECIFIC POPULATIONS Symptoms (DRESS)/Multiorgan Hypersensitivity . 8.1 Pregnancy 17.5 Lack of Interchangeability With Gabapentin Finally, gabapentin is likely used for RLS or PLMD in lieu of the costly, brand-name-only gabapentin enacarbil (Horizant), 39 despite package recommendations stating that these agents are not interchangeable due to varying pharmacokinetic properties. 39 Prior to market availability of gabapentin enacarbil, which does carry an FDA-approved Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. Table 1: FDA-Approved Indications for Pregabalin and Gabapentin: Indications. Pregabalin. Gabapentin. Neuropathic pain associated with diabetic peripheral neuropathy. x. Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Gabapentin tablets are indicated for: In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Indications and Usage for Gabapentin. • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. 2. Gabapentin Dosage and Administration. Different brands of gabapentin are not interchangeable and they are FDA approved for different conditions. Use only the brand and form of gabapentin your doctor has prescribed. Check your medicine each time you get a refill to make sure you receive the correct form. Neurontin and generic forms of Neurontin tablets may be broken into two pieces. You can take the second half for your next dose. Don't use the half-tablet beyond 28 days after the whole tablet was cut or broken. Carefully measure the liquid formulation of gabapentin using the measuring device that comes with the drug. Some of these individuals were taking higher than recommended doses of gabapentin for unapproved uses. When prescribing gabapentin, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 -----INDICATIONS AND USAGE----- NEURONTIN is indicated for: models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). In . This label FDA-Approved Indications. Gabapentin: Gabapentin is indicated for postherpetic neuralgia and serves as adjunctive therapy for managing partial seizures (with or without secondary generalization) in adults and pediatric patients aged 3 or older. FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in Gabapentinoid products include gabapentin, which is marketed under the name Neurontin
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |