Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
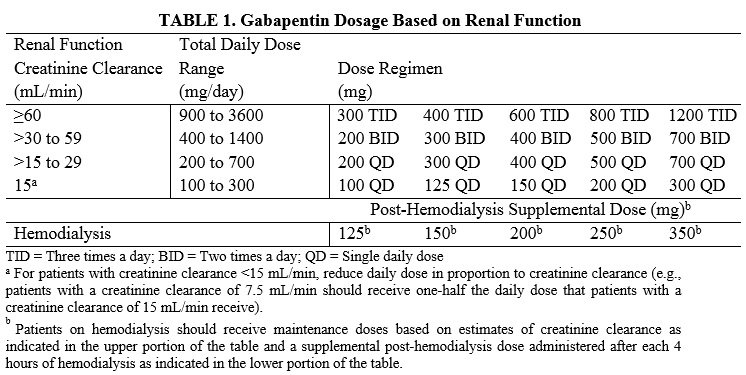
Gabapentin and pregabalin are FDA-approved for a variety of conditions, including seizures, nerve pain, and restless legs syndrome. Our evaluation shows that the use of these medicines, often A Cochrane review reported that 3 to 4 patients out of every 10 with either of these conditions experienced at least a 50% reduction in pain intensity when prescribed gabapentin at dosages of 1800mg-3600 mg/day (gabapentin encarbil: 1200mg-3600 mg/day). 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. A large primary care database review showed that in 2017, 21.8% of patients with a new prescription for gabapentin and 24.1% of patients with a new prescription for pregabalin received a concomitant prescription, primarily for opioids. 2 In response to increasing reports of respiratory depression, the Medicines and Healthcare Products Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. We reviewed the published comparative effectiveness literature for gabapentinoids for pain as well as all trials (published and unpublished) used by the FDA for the approval of the five pain indications for these agents (one for gabapentin, four for pregabalin). Bulk Drug Substances Currently Under Review. FDA-2018-N-4626-0051 FDA-2018-N-4626-0084 Gabapentin/trazodone HCl dog, cat FDA-2018-N-4626-0128 ferret, small mammals, bird, (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin's antinociceptive and antiseizure effects in animal models. In vitro, pregabalin reduces the calcium-dependent release of several neurotransmitters, possibly by modulation of calcium channel function. Aside from the commercial marketing, gabapentin was promoted via a “seeding” clinical trial called the Dosing to Efficacy with Neurontin: Study of Titration to Effect, Profile of Safety (STEPS) trial. 41 Seeding trials ultimately aim to promote medications that are either under FDA review or recently FDA-approved by allowing recruited FDA is warning that serious, life-threatening, and fatal respiratory depression has been reported with the gabapentinoids, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica We reviewed the published comparative effectiveness literature for gabapentinoids for pain as well as all trials (published and unpublished) used by the FDA for the approval of the five pain indications for these agents (one for gabapentin, four for pregabalin). Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in patients who use gabapentanoids with opioid pain medicines or other drugs that depress the gabapentin. After an oral dose of GE the resulting plasma concentration of gabapentin is greater compared to approved gabapentin products.. The systemic exposure of GE at 600mg/day is similar to the systemic exposure of Neurontin at approved doses of Neurontin (1200-1800mg/day) Please refer to Clinical Review, 02/10/2010. We reviewed the published comparative efectiveness literature for gabapentinoids for pain as well as all trials (published and unpublished) used by the FDA for the approval of the five pain indications for these agents (one for gabapentin, four for pregabalin). Active Ingredient: Gabapentin . Dosage Form: Tablet . Route: Oral . Strengths: 300 mg, 450 mg, 600 mg, 750 mg, 900 mg. Recommended Studies: Three in vivo bioequivalence studies with Title: 21-397.pdf Neurontin Pharmacology Review Created Date: 4/29/2004 10:03:22 AM Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |