Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
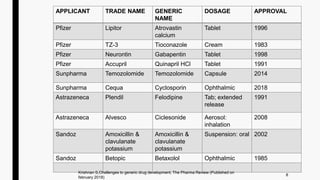
Extended-release Tablets: gabapentin enacarbil: 300 mg and 600 mg: 022399: 1: 2019-04-29: This potential generic entry date is based on patent ⤷ Try for Free. Neurontin is a drug owned by Viatris Specialty Llc.It is protected by 4 US drug patents filed in 2013 out of which all have expired. Based on its patents and exclusivities, its generic launch date is estimated to be Nov 28, 2022. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg GABARONE capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Generic Gralise Availability. Last updated on Mar 13, 2025. Gralise is a brand name of gabapentin, approved by the FDA in the following formulation(s): GRALISE (gabapentin - tablet;oral) Manufacturer: ALMATICA Approval date: January 28, 2011 Strength(s): 300MG , 600MG ; Manufacturer: ALMATICA Approval date: April 18, 2023 Piscataway, NJ, October 10, 2023–Camber Pharmaceuticals is pleased to announce the addition of Gabapentin Capsules, USP to its current portfolio. Gabapentin Capsules, USP are indicated for: Gabapentin Capsules, USP are available in 100, 300, and 400 mg strengths in 500 count bottles. Gabapentin’s anticipated release date is in the first quarter of 2024. Several generic applications have been filed for Gabapentin. The first generic version for Gabapentin was by Actavis Elizabeth Llc and was approved on Sep 12, 2003. And the latest generic version is by Strides Pharma Global Pte Ltd and was approved on Dec 20, 2024. Gabapentin was originally marketed under the brand name Neurontin. Since it became generic, it has been marketed worldwide using over 300 different brand names. [1] An extended-release formulation of gabapentin for once-daily administration was introduced in 2011, for postherpetic neuralgia under the brand name Gralise. [155] Launch of generic Gralise (Once Daily Gabapentin tablets) 800.553.1783; Need a specific resource? Launch of generic Gralise (Once Daily Gabapentin tablets) Generic Horizant Availability. Last updated on Mar 13, 2025. Horizant is a brand name of gabapentin enacarbil, approved by the FDA in the following formulation(s): HORIZANT (gabapentin enacarbil - tablet, extended release;oral) Manufacturer: AZURITY Approval date: April 6, 2011 Strength(s): 600MG ; Manufacturer: AZURITY Approval date: December On January 25, 2024, Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. — Gralise is also available as 450 mg, 750 mg and 900 mg tablets. Gralise is approved for the management of postherpetic neuralgia (PHN). January 25, 2024 - Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. Camber Pharmaceuticals is pleased to announce the addition of Gabapentin Tablets (generic for Gralise®)to its current portfolio. Camber already offers Gabapentin Tablets and Capsules (generic for Neurontin®). Generics for Gralise® and Neurontin® are not interchangeable. Gabapentin Extended-Release Tablets. temperature between 15 and 30 degrees C (59 and 86 degrees F). Get rid of any unused medication after the expiration date. New Drug Review Generic Name: (gabapentin) Trade Name: (Gralise®) Manufacturer: Depomed Inc. Food and Drug Administration Approval Date: January 28, 2011 Product Launch Date: October 10, 2011 Overview/Summary Gralise® (gabapentin) is a new once-daily formulation of the antiepileptic drug gabapentin that is Food Gralise (gabapentin) is an extended release, tablet formulation of the approved antiepileptic agent gabapentin, indicated for the once-daily treatment of post-herpetic neuralgia (PHN). Development timeline for Gralise It’s important to note that while generic Gabapentin is a more affordable choice, it is equally as effective as the brand name version. Generic medications undergo rigorous testing to ensure their safety and efficacy, making them a reliable alternative. In summary, generic Gabapentin is a cost-effective alternative to the brand name version. Directorate General Of Health Services Ministry of Health & Family Welfare, Government of India Zydus is the first company to receive final approval for generic Gabapentin Tablets (Once-Daily), 300 mg and 600 mg. Following approval, the product will be launched immediately in the US. Gabapentin is indicated for the management of Postherpetic Neuraligia (PHN).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |