Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 | 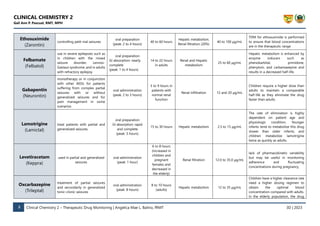 |
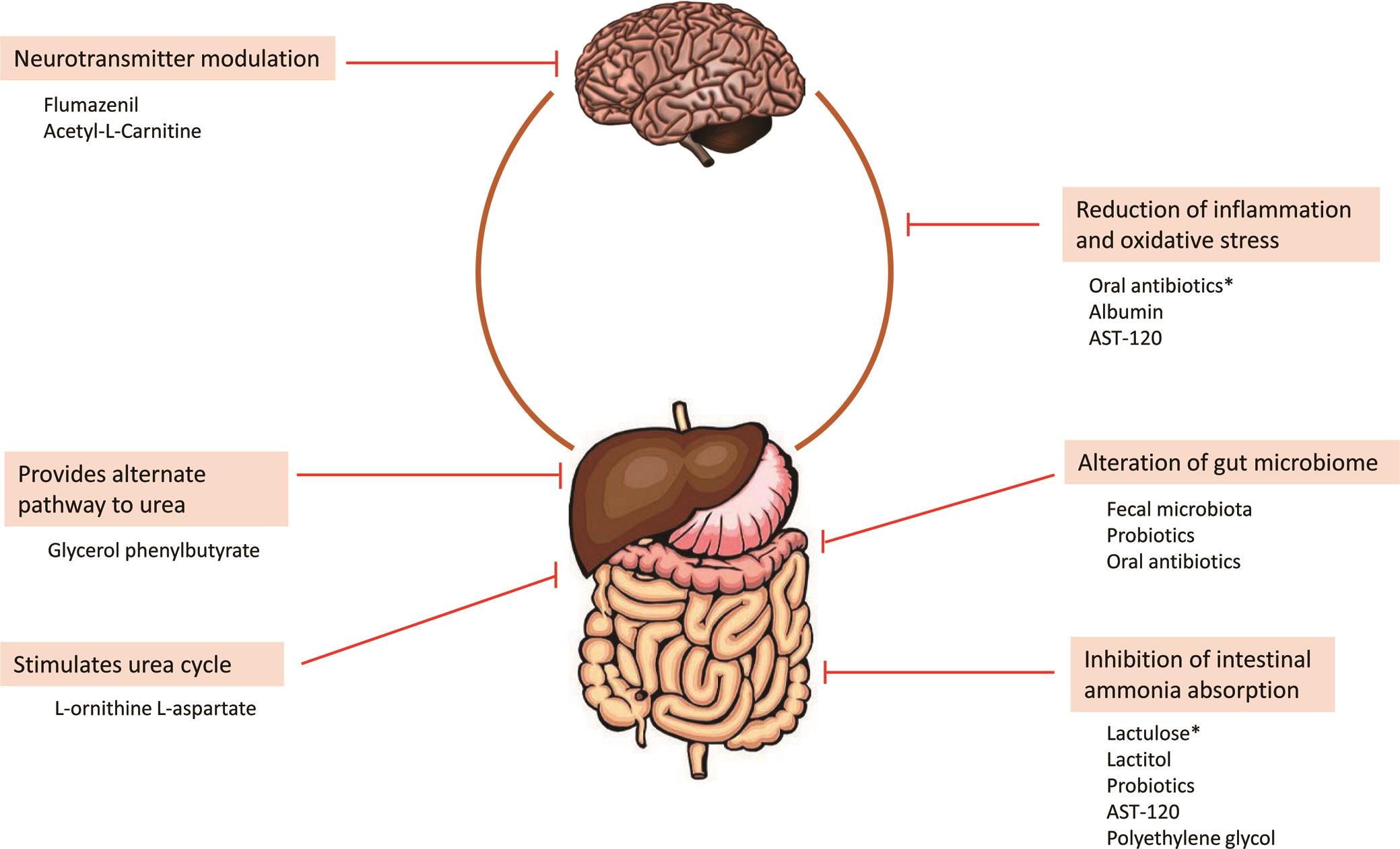
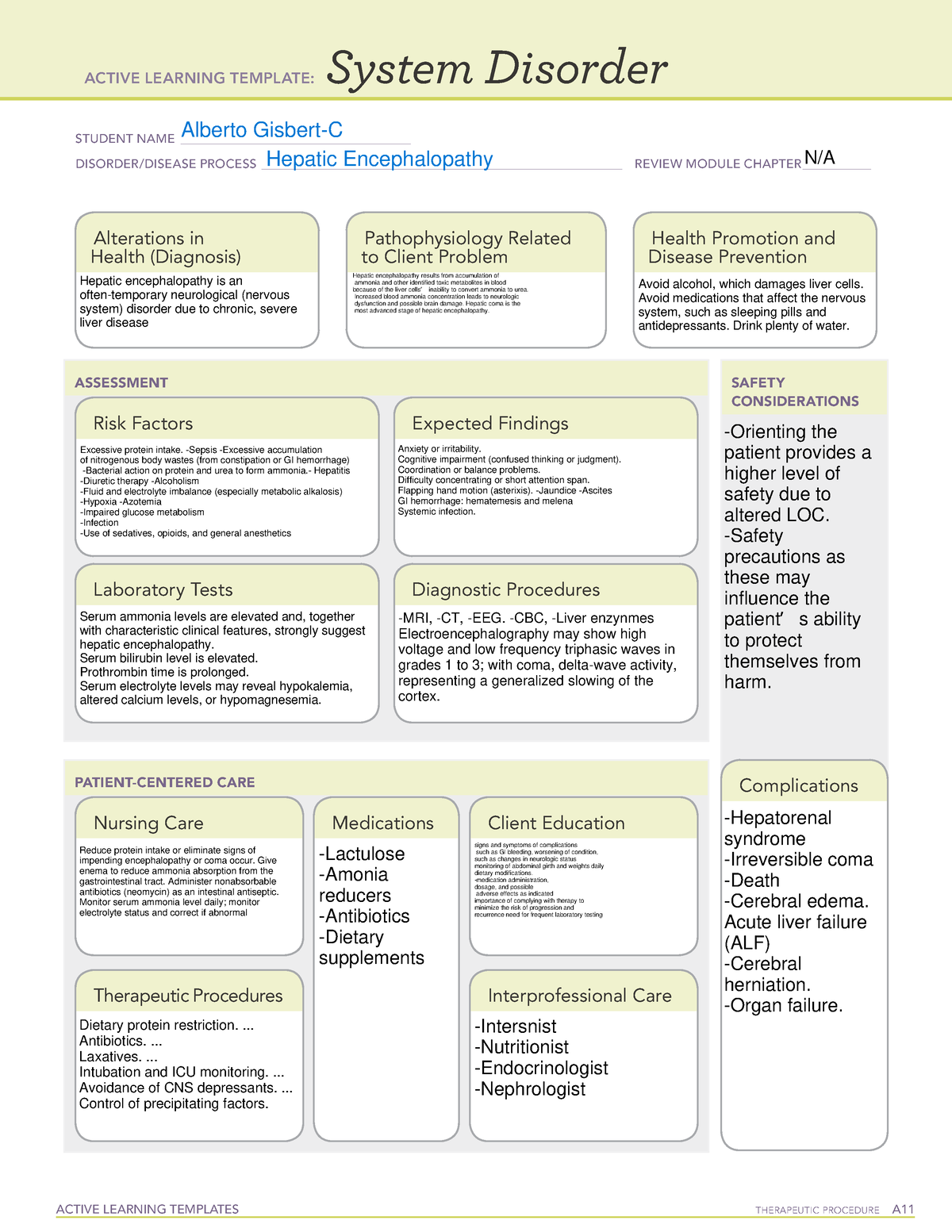
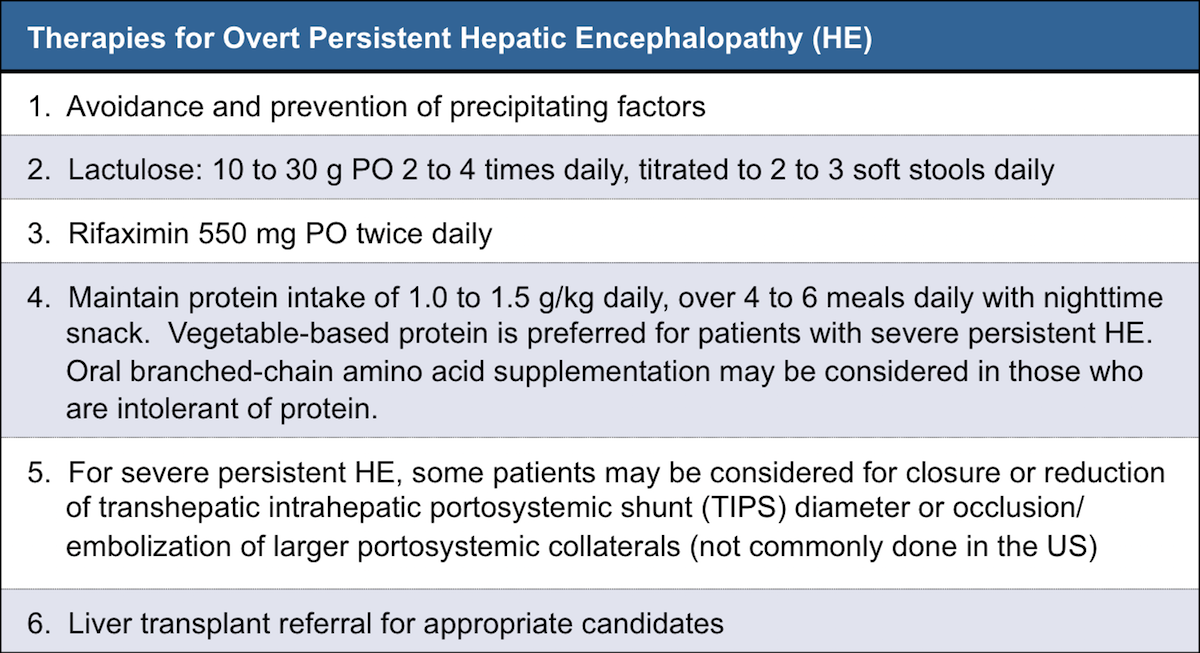
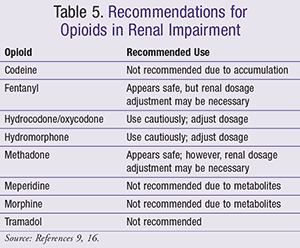
Human experiments demonstrate that the clearance of LTG depends on the severity of hepatic impairment. 25 One group noted the need to reduce the dose by 50–75% in patients with liver cirrhosis corresponding to Child–Pugh Grade B or C, respectively. 26 In vitro experiments by Furlan et al. 27 demonstrated no significant changes in the Herein, we report a gabapentin-induced hepatocellular injury in a patient without another identifiable cause for acute liver injury. Discontinuing gabapentin resulted in rapid reversal improvement in hepatocellular injury. Keywords: drug-induced liver injury; gabapentin; hepatotoxicity. While there are no cures for the late-stage liver disease there are various treatment options including gabapentin and cirrhosis of the liver. One of the main goals of cirrhosis treatment is to ease the symptoms. Some options include avoiding alcohol, a low-salt diet, and weight loss. Identify the appropriate indications for gabapentin therapy, including neuropathic pain, partial onset seizures, restless legs syndrome, and other relevant neurological and psychiatric conditions. Hepatic impairment No adjustments are required for hepatic impairment. Renal impairment Clearance of gabapentin is approximately proportional to creatinine clearance. Dose adjustment is not necessary for patients with creatinine clearance (CrCl) ≥60 mL/min. For patients with CrCl 30-59 In patients with liver disease, impaired hepatic function, reduced hepatic blood flow, and portosystemic shunting reduce first‐pass metabolism, thereby increasing the proportion of drug that is bioavailable and increasing the risk for toxicity. Table 1. Important Considerations Regarding Analgesia Prescribing for Patients With Cirrhosis. Herein, we report a gabapentin-induced hepatocellular injury in a patient without another identifiable cause for acute liver injury. Discontinuing gabapentin resulted in rapid reversal improvement in hepatocellular injury. Keywords: gabapentin, hepatotoxicity, drug-induced liver injury. Start at 15mg PO ON and titrate slowly to maximum dose 30mg ON. Avoid if patient has renal impairment. May help to stimulate appetite. Sedating effect greater at lower dose. Caution use in severe liver impairment, reduce dose in renal impairment. Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported. Severe complications from analgesia in these patients include hepatic encephalopathy, hepatorenal syndrome, and gastrointestinal bleeding, which can result in substantial morbidity and even death. In general, acetaminophen at reduced dosing is a safe option. Gabapentin (Neurontin) usually isn’t bad for your liver or kidneys. In most cases, it has little effect on these organs. In rare instances, gabapentin can cause DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. This is a severe allergic reaction that can cause damage to major organs, including the liver and kidneys. All pain medications should be titrated carefully to achieve safe and adequate pain relief in patients with hepatic impairment. Cirrhosis is defined as permanent liver fibrosis secondary to damage or injury. Liver and renal functions were impaired by gabapentin; where hepatotoxicity was associated by an imbalance in the redox status. However, magnesium only elevated blood urea nitrogen (BUN). Question. I have a patient with trigeminal neuralgia who was taking 1600 mg of gabapentin and had serious elevations of liver function tests (aspartate transaminase 258 U/L, alanine transaminase Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided The halflife of acetaminophen can be nearly doubled in hepatic impairment. 19 Despite this, PO acetaminophen can still be used in severe hepatic impairment at a reduced dose and provided there are no additional risk factors for toxicity. 7,20 Although IV acetaminophen is contra-indicated by the manufacturers in severe hepatic impairment, it is Gabapentin is not metabolized by the liver. Instead, it is excreted unchanged in your kidneys after circulating in your blood. Gabapentin affects nerves and chemicals in your body that are involved in some types of pain and in seizures. PHARMACOLOGICAL IMPACT OF HEPATIC IMPAIRMENT The liver is the main site for the metabolism of most drugs. However, hepatic reserve is large and, generally, there must be severe hepatic impairment or decompensation for the overall intrinsic activity or capacity of the metab-olizing enzymes to be altered to a clinically important extent. The LiverTox, an authoritative resource for drug-induced liver injury, classifies these 2 agents as class C, indicating that clinically apparent liver injury is uncommon. 55, 56 Mechanistically, both agents are likely safe to use in hepatic impairment, as they are primarily renally eliminated in an unchanged form and undergo negligible hepatic
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |