Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
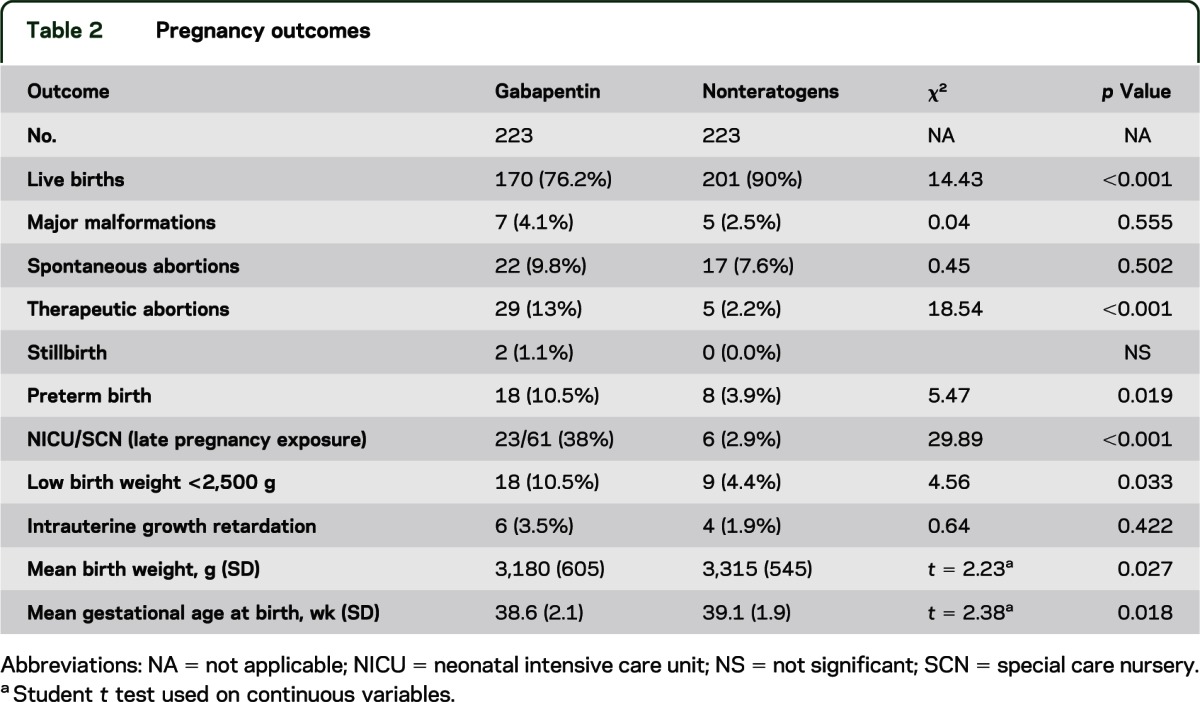
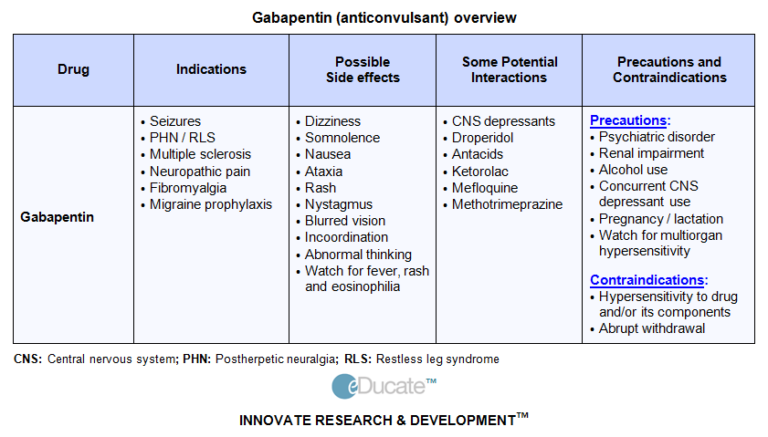
We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. Purpose. Pain in the postpartum period is common and considered by many individuals to be both problematic and persistent 1.Pain can interfere with individuals’ ability to care for themselves and their infants, and untreated pain is associated with risk of greater opioid use, postpartum depression, and development of persistent pain 2. The American College of Obstetricians and Gynecologists (ACOG) has released guidelines on psychiatric medication used by women during pregnancy and lactation. The use of psychotropic medications In this large population-based study, we did not find evidence for an association between gabapentin exposure during early pregnancy and major malformations overall, although there was some evidence of a higher risk of cardiac malformations. Maternal use of gabapentin, particularly late in pregnancy Results: We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. There was a higher risk of preterm birth among women exposed to gabapentin either late (RR, 1.28 [1.08–1.52], p < 0.01) or both early and late in pregnancy (RR, 1.22 [1.09–1.36], p < 0.001), SGA among women exposed to gabapentin early (1.17 [1.02–1.33], p = 0.02), late (1.39 [1.01–1.91], p = 0.05), or both early and late in pregnancy Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, gabapentin, topiramate, valproate, calcitonin gene-related protein antagonist monoclonal antibodies, feverfew, melatonin All ACOG committee members and authors have submitted a conflict of interest disclosure statement related to this published product. Any potential conflicts have been considered and managed in accordance with ACOG’s Conflict of Interest Disclosure Policy. The ACOG policies can be found on acog.org. For products jointly developed with other Number 776 (Reaffirmed 2021). Committee on Obstetric Practice. Society for Maternal-Fetal Medicine. This Committee Opinion was developed by the American College of Obstetricians and Gynecologists' Committee on Obstetric Practice in collaboration with committee member Alison G. Cahill, MD, MSCI, and the Society for Maternal-Fetal Medicine in collaboration with member T. Flint Porter, MD. If cessation is necessary, tricyclic antidepressants should not be stopped suddenly; weaning the patient by 10–25 mg every few days is indicated. Gabapentin, used to manage neuropathic pain disorders, is the most studied and used anticonvulsant for vulvodynia 4. Dosage can be increased over time from 300 mg total daily to a maximum dose of Prenatal exposure to pregabalin is associated with an increased risk of congenital anomalies and long-term neurodevelopmental outcomes while gabapentin exposure was associated with an increased risk of preeclampsia, preterm birth and small-for-gestational age. There was an increased risk of preterm birth among women exposed to gabapentin either late (RR=1.28 [CI 1.08-1.52], p < 0.01) or both early and late in pregnancy (RR=1.22 [1.09-1.36], p < 0.001). With maternal doses up to 2.1 g/day, estimated doses for fully breastfed infants are 0.2 to 1.3 mg/kg/day (equivalent to 1.3 to 3.8% of the maternal weight-adjusted dose). An expert panel has deemed this drug is an acceptable choice for refractory restless leg syndrome during lactation. There was a higher risk of preterm birth among women exposed to gabapentin either late (RR, 1.28 [1.08-1.52], p < 0.01) or both early and late in pregnancy (RR, 1.22 [1.09-1.36], p < 0.001), SGA Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. When treating neuropathic pain in a woman who is pregnant, the use of gabapentinoids (e.g. gabapentin) or an antiepileptic drug (AED) (e.g. levetiracetam, lamotrigine) is a last line option. This is due to the limited availability of data for safe use during pregnancy. Other options should be trialled first. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. Gabapentin has not been categorized as yet within the FDA Pregnancy Risk Classification System. Antidepressants are labeled with category D classification. For unrelenting severe pain, pregnant women may receive opioid medications, although the prescribing physician must be cautious with the medication regimen to avoid opioid withdrawal in the
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |