Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
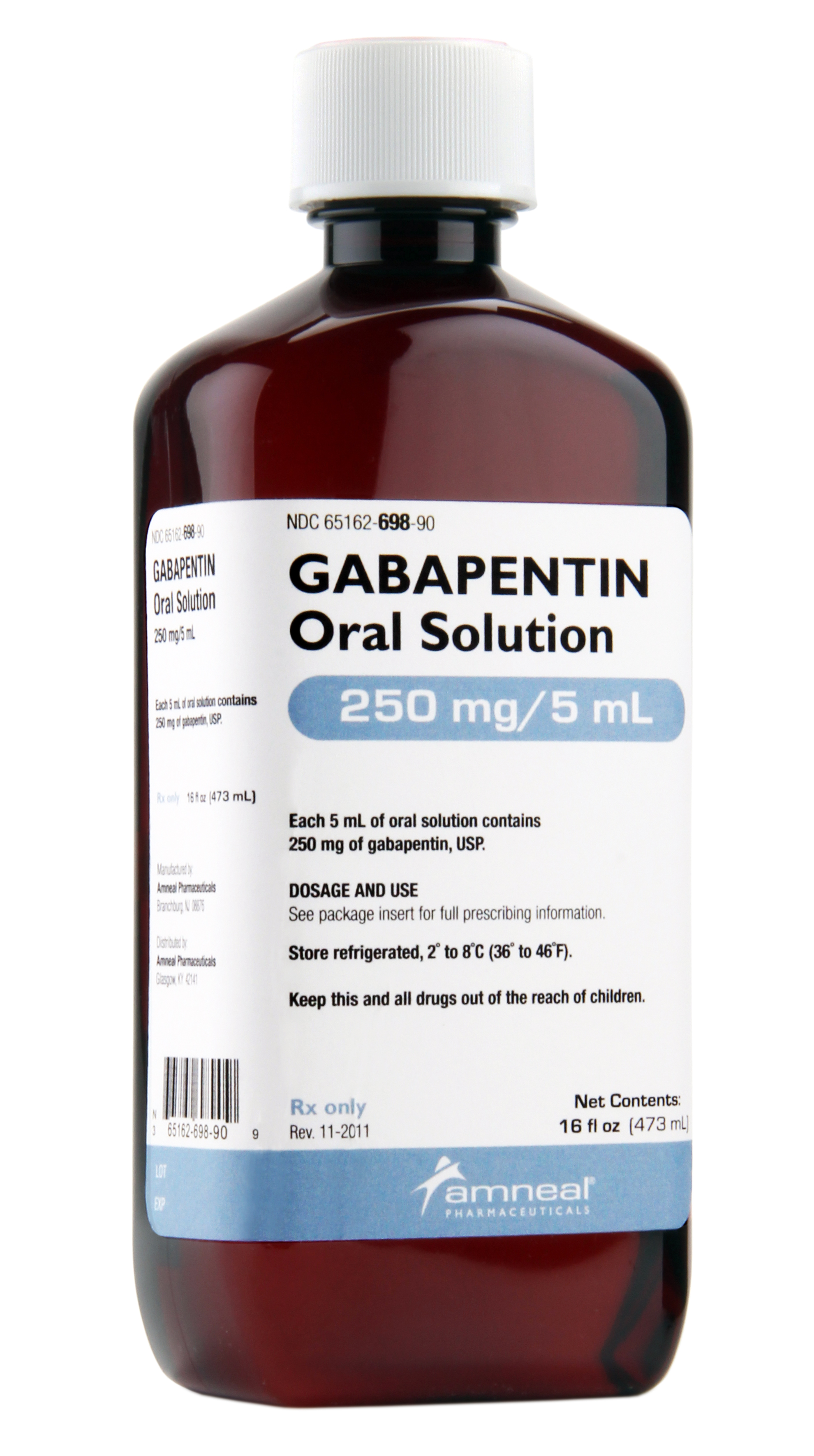
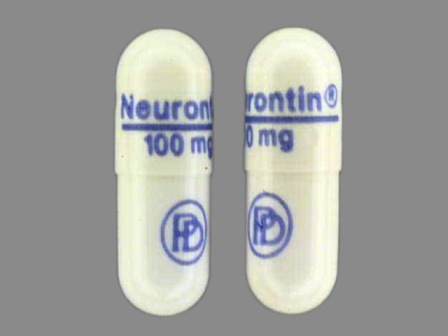
Gralise (gabapentin) is an extended release, tablet formulation of the approved antiepileptic agent gabapentin, indicated for the once-daily treatment of post-herpetic neuralgia (PHN). Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances. Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or One such drug, gabapentin (Neurontin), received approval by the U.S. Food and Drug Administration (FDA) in 1993 as an adjunct medicine for partial seizures and additional FDA approval in 2002 for Gabapentin — an anti-seizure drug also approved for nerve pain related to shingles — is now among the most widely prescribed drugs in the country. It's used off-label to treat menopausal Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. FULL PRESCRIBING INFORMATION . 1 INDICATIONS AND USAGE . 1.1 Treatment of Restless Legs Syndrome . HORIZANT (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of Gabapentin is FDA approved for pain management of a limited number of neuropathic pain conditions; Gabapentin is widely used off-label for various chronic pain conditions and for the treatment of acute pain, making it now one of the most commonly described analgesic drugs An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about FDA Approved Labeling Text dated 03/01/2011 Page 2 . particular, gabapentin prevents pain-related responses in several models of neuropathic pain in rats or mice (e.g. spinal nerve ligation Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. Gabapentin and pregabalin are FDA-approved for a variety of conditions, including seizures, nerve pain, and restless legs syndrome. Our evaluation shows that the use of these medicines, often The tricyclic antidepressant amitriptyline is FDA approved for depression and is also frequently used for the treatment of anxiety, neuropathic pain, and potentially for chronic cough. 27,28 At Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in Gabapentinoid products include gabapentin, which is marketed under the name Neurontin FDA is warning that serious breathing difficulties may occur in patients using gabapentin or pregabalin who have Gabapentin was first approved in 1993 and pregabalin was first approved in 2004 Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |