Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
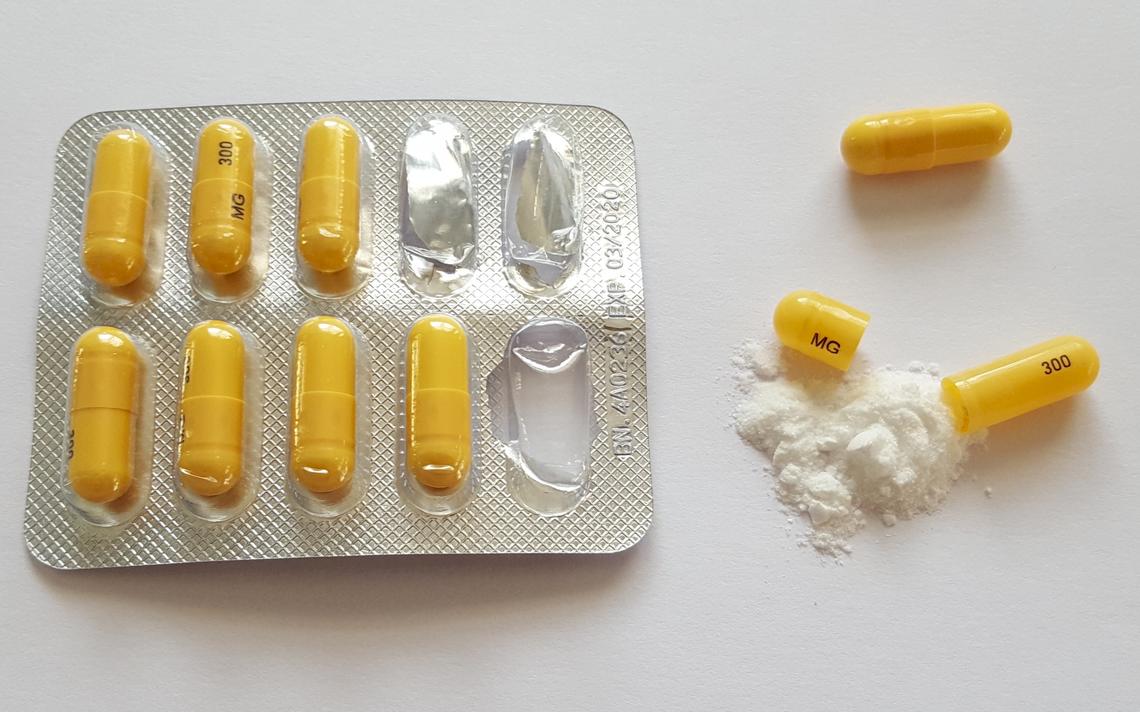
Gabapentin is a medication for nerve pain and seizures. It’s not a controlled substance by the federal government, but some states classify it as a schedule V substance due to its risks of addiction and overdose. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways prescription drug pursuant to Wis. Stat. § 961.385 (1) (ag). Gabapentin is now listed in Wis. Admin. Code § CSB 4.03 (2) as a monitored prescription drug effective September 1, 2021. Gabapentin Has Not Been Scheduled as a Controlled Substance Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states due to its potential for abuse and diversion. Learn which states control gabapentin, why it is regulated, and how it can interact with opioids and other drugs. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin isn’t a controlled substance or narcotic on the federal level, but several states have passed laws to make it a Schedule V controlled substance. Gabapentin has risks and adverse effects, especially when combined with some other substances. Gabapentin is an anticonvulsant medicine that can treat nerve pain and restless legs syndrome, but it is not a painkiller or an opioid. It can be misused and abused, and it can cause serious breathing problems with other drugs. Learn more about gabapentin brands, dosage, and alternatives. Although the anticonvulsant is not considered a controlled substance, some state legislation focuses on monitoring the use of or reclassifying it. The FDA approved gabapentin in 1993 as a non-controlled substance and it has remained a non-controlled substance at the federal level. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly The petition also pointed to studies suggesting that both gabapentin and pregabalin, which is marketed as Lyrica and is already classified as a Schedule 5 controlled substance, pose an addiction risk for patients with current or past substance abuse. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). For controlled substance licensure, the rule changes require a designated prescriber to have a controlled substance license for a health facility if substances are stored there without an on-site pharmacy or an automated device stocked by a pharmacy, provide an exception to licensure for an emergency kit that contains controlled substances Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. 11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |