Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
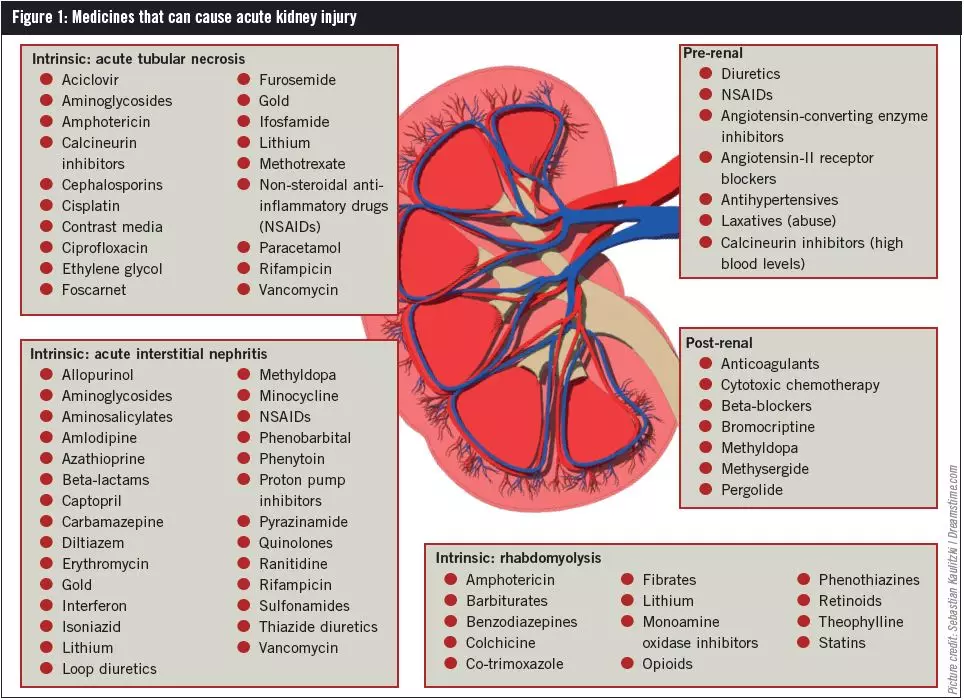 |  |
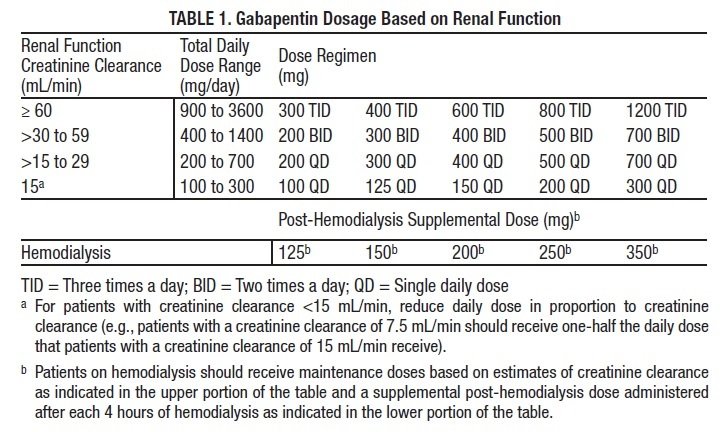
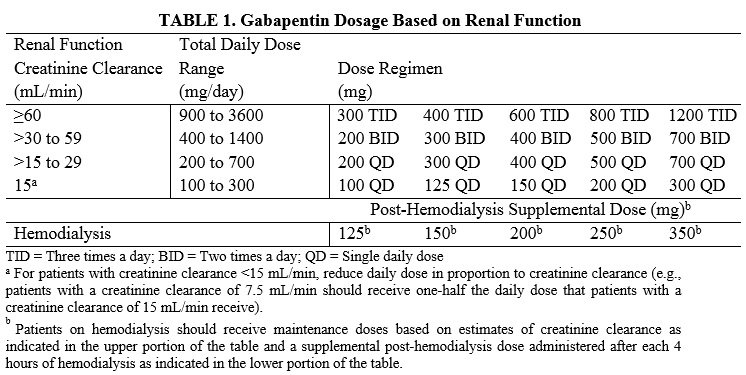
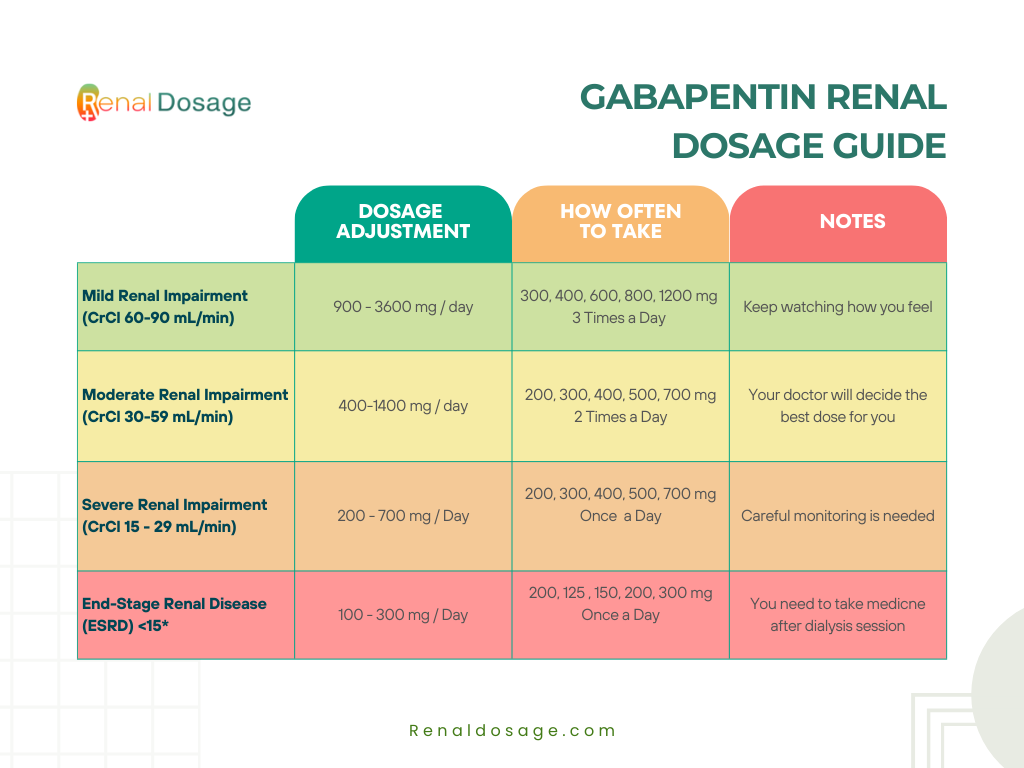
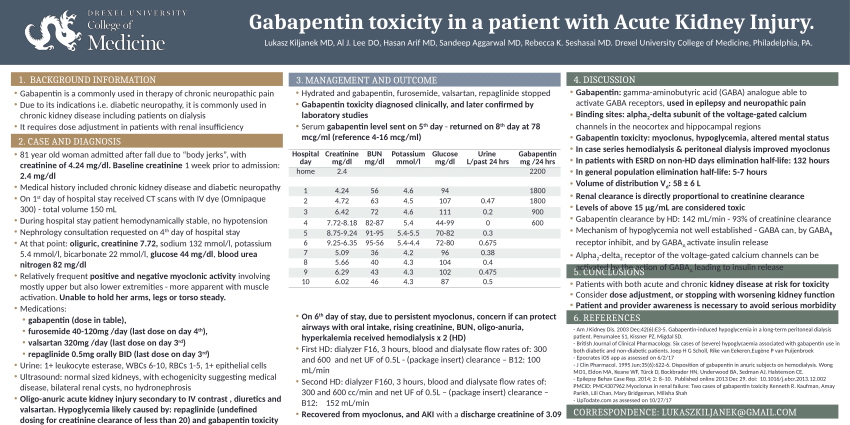
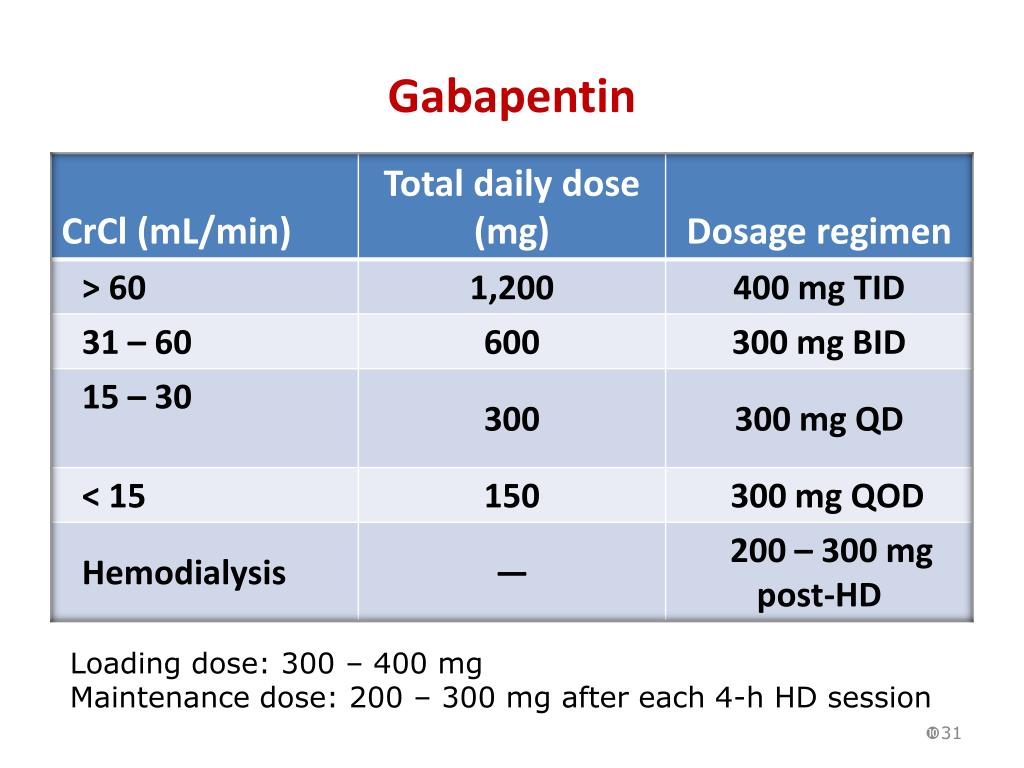
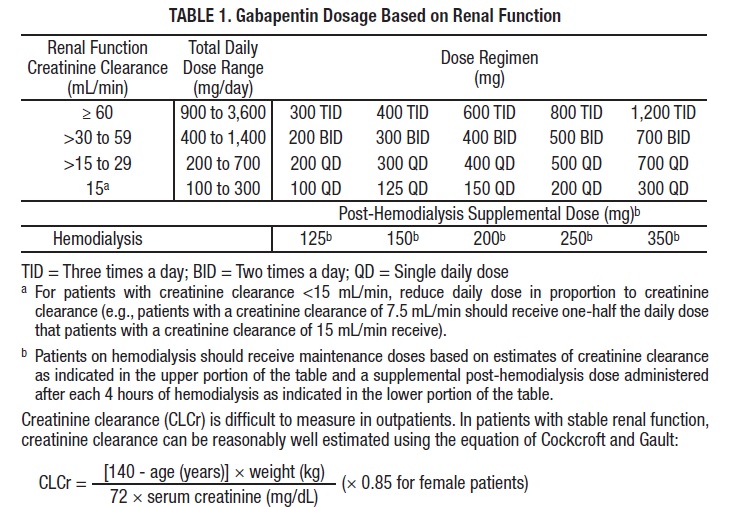
Guidelines for medicines optimisation in patients with acute kidney injury 3 1. Introduction Acute kidney injury (AKI) is the sudden loss of kidney function over a period of hours or days. Since the kidneys are one of the major excretory pathways for the removal of drugs from the Gabapentin and pregabalin are commonly used for neuropathic pain in CKD patients but are not fully understood as this population remains excluded from efficacy and safety trials. Renal adjustments for the gabapentinoids are prodigiously recommended in the literature. Acute kidney injury is a clinical syndrome characterized by a rapid decline in glomerular filtration rate and resultant accumulation of metabolic waste products. Acute kidney injury is associated Gabapentin is actually toxic to the kidneys. Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its well recieved pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Acute kidney injury (AKI) is a common complication in critically ill patients with various etiologies and risk factors. Admissions for AKI have increased in the past decade, 1 compounded by COVID-19 critical illness, 2 and have surpassed the incidence of hospitalizations from end-stage renal disease (ESRD). 3 The varying etiologies of AKI create complex interactions when evaluating changes in Gabapentin, a home medication, was increased from 600 mg TID to 800 mg TID. Unfortunately, patient experienced acute kidney injury in setting of IV contrast administration; her creatinine increased from 0.72 to 3.2 over 5 days. On day 4, patient developed depressed mental status with myoclonus. Higher-dose gabapentinoids (gabapentin >300 mg/d or pregabalin >75 mg/d) versus lower-dose gabapentinoids (gabapentin ≤300 mg/d or pregabalin ≤75 mg/d). Outcomes The primary composite outcome was the 30-day risk of a hospital visit with encephalopathy, a fall, or a fracture or a hospitalization with respiratory depression. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. The short answer is: yes, gabapentin can be problematic for individuals with kidney failure and chronic kidney disease (CKD). While gabapentin is often prescribed for pain management, particularly nerve pain, and sometimes for seizures, its primary elimination pathway is through the kidneys. In most cases, gabapentin doesn’t hurt the liver or kidneys, though proper dosing is important to prevent side effects. Learn how gabapentin affects the liver and kidneys here. Pain is one of the most common and distressing symptoms among patients with chronic kidney disease (CKD) . The prevalence of pain has been associated with substantially lower health-related quality of life and greater psychosocial distress, insomnia, and depressive symptoms [ 2-9 ]. Rational dosing of gabapentin and pregabalin in chronic kidney disease normal renal function on maximum recommended dosing yielded concentrations of 5–8 mg/L for gabapentin and ~ 2.8–8.2 mg/L for pregabalin. 22–25 The elimination half-lives of gabapentin and pregabalin are prolonged with renal impairment leading up to accumulation with Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Conclusion: All doctors need to be aware of the need to review the indications for gabapentin use during periods of acute illness, especially with regard to renal impairment. Since gabapentin is cleared solely by renal excretion, dosing requires consideration of the patient's renal function. Myoclonic activity may occur as a complication of gabapentin toxicity, especially with acute kidney injury or end-stage renal disease. Gabapentinoids are opioid substitutes whose elimination by the kidneys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. With a growing chronic kidney disease epidemic,22, 23 an increasing number of patients with chronic kidney disease will be exposed to gabapentin. This study demonstrates that gabapentin dosage for patients with chronic kidney disease has been insufficiently adjusted and that the risk of gabapentin toxicity has been underrecognized. Since gabapentin is cleared solely by renal excretion, dosing requires consideration of the patient's renal function. Myoclonic activity may occur as a complication of gabapentin toxicity, especially with acute kidney injury or end-stage renal disease. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |