Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
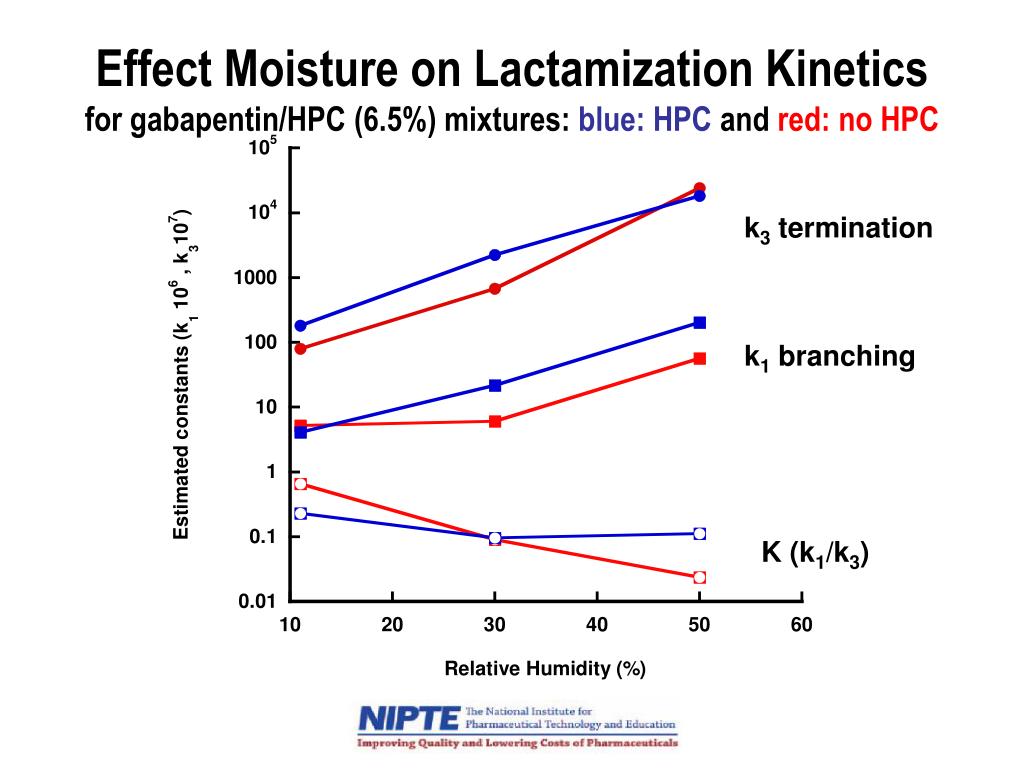 |  |
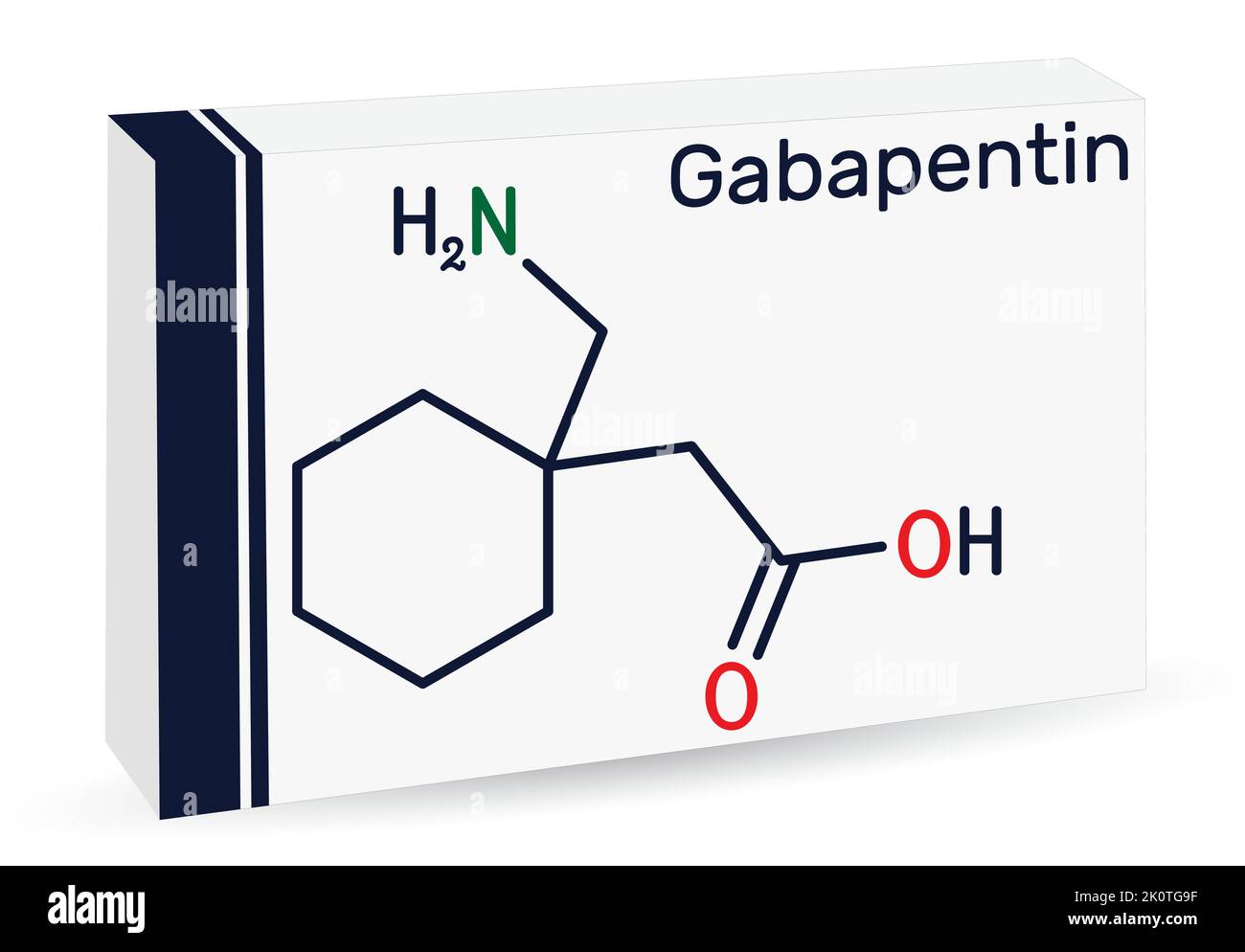
Gabapentin has saturable, non-linear absorption kinetics, where bioavailability decreases as the dose increases. 10 Following oral administration, gabapentin’s bioavailability is 60%, 47%, 34%, and 33%, following 900, 1200, 2400, and 3600 mg/day in 3 divided doses, respectively. Gabapentin is a new, water-soluble, antiepileptic agent with properties of an amino acid. This drug is rapidly absorbed and exhibits dose-dependent bioavailability as a result of a saturable transport mechanism. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Gabapentin was stopped with pregabalin started at the next . scheduled dose in the abrupt conversion group. The other group gradually . transitioned by reducing the dose of gabapentin by 50% with Gabapentin degrades directly to gabapentin-lactam (gaba-L) in the solid state. The objective of this study was to formulate a drug degradation model that accounted for the environmental storage conditions and mechanical stress (prior to storage) on lactamization kinetics. The effects of mechanical stress on drug degradation kinetics were determined by milling gabapentin in a FRITSCH Planetary Gabapentin has a narrow absorption window, an approximate half-life of 6 hours and is usually taken in multiple daily doses. As a result, it was considered appropriate for the developed gastroretentive system 21,22. Gabapentin is mainly used as an anticonvulsant agent and for neuropathic pain 23. The pharmacokinetics of gabapentin are a little unique in that gabapentin has an inverse dose dependent absorption. What does this mean? This means that as you increase the dose, you actually get less (percentage) of the medication absorbed. This study analyzes pH-dependent reaction kinetics and pathways of gabapentin (GBP), an aliphatic primary amine with an additional carboxylic acid group. The transformation pathway was elucidated applying a novel approach using isotopically labeled ozone ( 18 O) and quantum chemistry calculations. Objective: Gabapentin immediate release (GBP-IR), gabapentin gastric retentive (GBP-GR), and the prodrug gabapentin enacarbil extended release formulation (GEn) have been approved for management of postherpetic neuralgia (PHN) in adults. Gabapentin is available in two extended-release for-mulations in addition to the immediate release: a gas-tric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neural-gia. The gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009; 139: 380–392. Crossref. PubMed. Gabapentin degrades directly to gabapentin-lactam (gaba-L) in the solid state. The objective of this study was to formulate a drug degradation model that accounted for the environmental storage conditions and mechanical stress (prior to storage) on lactamization kinetics. More recently, pre-morning dose serum GBP concentrations in 228 patients with epilepsy were studied 6 which showed that individual patients produced a significant correlation between serum level and dose within the daily range of 400–4800 mg. Up to 6 g per day GBP were administered to patients in another recent study 7 and blood was collected for drug determination which showed that serum Kinetics of gabapentin uptake were determined by selecting a time point within a linear range (1 min) and then determining influx at different concentrations of drug. The rate of drug transport by LAT1 and other transport processes (pmoles/min/million cells) was plotted against gabapentin concentration (μM). Although not directly involved in Ca 2+ transport, Ca v β and Ca v α2δ are critical for VGCC function by regulating kinetics and cell surface expression of these channels. Gabapentinoids, including gabapentin and pregabalin, are extensively used for treatment of neuropathic pain, restless legs syndrome, and focal seizures. There are AEDs of which adverse effects outweigh beneficial effects, such as valproic acid, carbamazepine, phenytoin, or phenobarbital and there are AEDs in which beneficial effects dominate over mitochondrial toxic effects, such as lamotrigine, levetiracetam, gabapentin, or zonisamide. Pregabalin and gabapentin both show dose-response relationships in the treatment of postherpetic neuralgia and partial seizures. For neuropathic pain, a pregabalin dosage of 450 mg/day appears to reduce pain comparably to the predicted maximum effect of gabapentin. Carbamazepine (autoinduction), gabapentin (saturable GI tract transport), valproic acid/divalproex sodium/valproate sodium (saturable albumin binding), zonisamide (saturable erythrocyte binding), and phenytoin (saturable CYP2C9 biotransformation) exhibit nonlinear kinetics, as shown in Table 2.62 Zonisamide is extensively bound to erythrocytes The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |