Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
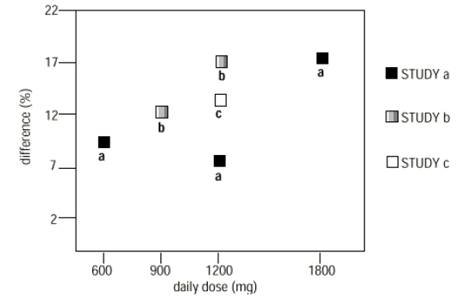
3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Gabapentin oral solution is indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Moreover, because gabapentin oral solution causes somnolence and dizziness [see Warnings and Precautions (5.4)], patients should be advised not to operate complex machinery until they have gained sufficient experience on gabapentin oral solution to assess whether gabapentin oral solution impairs their ability to perform such tasks. The recommended maintenance dose of gabapentin oral solution in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin oral solution may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of somnolence caused by NEURONTIN, can be imperfect. The duration of . 2.1 Dosage for Postherpetic Neuralgia . In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. maintenancedose of NEURONTIN in patients 3 to 4 years of age is 40mg/kg/day,given in three divided doses. The recommended maintenancedose of NEURONTIN in patients 5 to 11 years of age is 25mg/kg/day to 35mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or Gabapentin oral solution is indicated for: In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times talking with your healthcare provider. Taking gabapentin with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse. • Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin affects you. Gabapentin can slow your thinking and motor skills. Patients taking Gabapentin Oral Solution should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 GABAPENTIN- gabapentin solution Advagen Pharma Ltd-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Administer gabapentin capsules orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin capsules dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). What is Gabapentin Oral Solution? Gabapentin Oral Solution is a prescription medicine used to treat: zPain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. zPartial seizures when taken together with other medicines in adults and children 3 years of
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |