Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
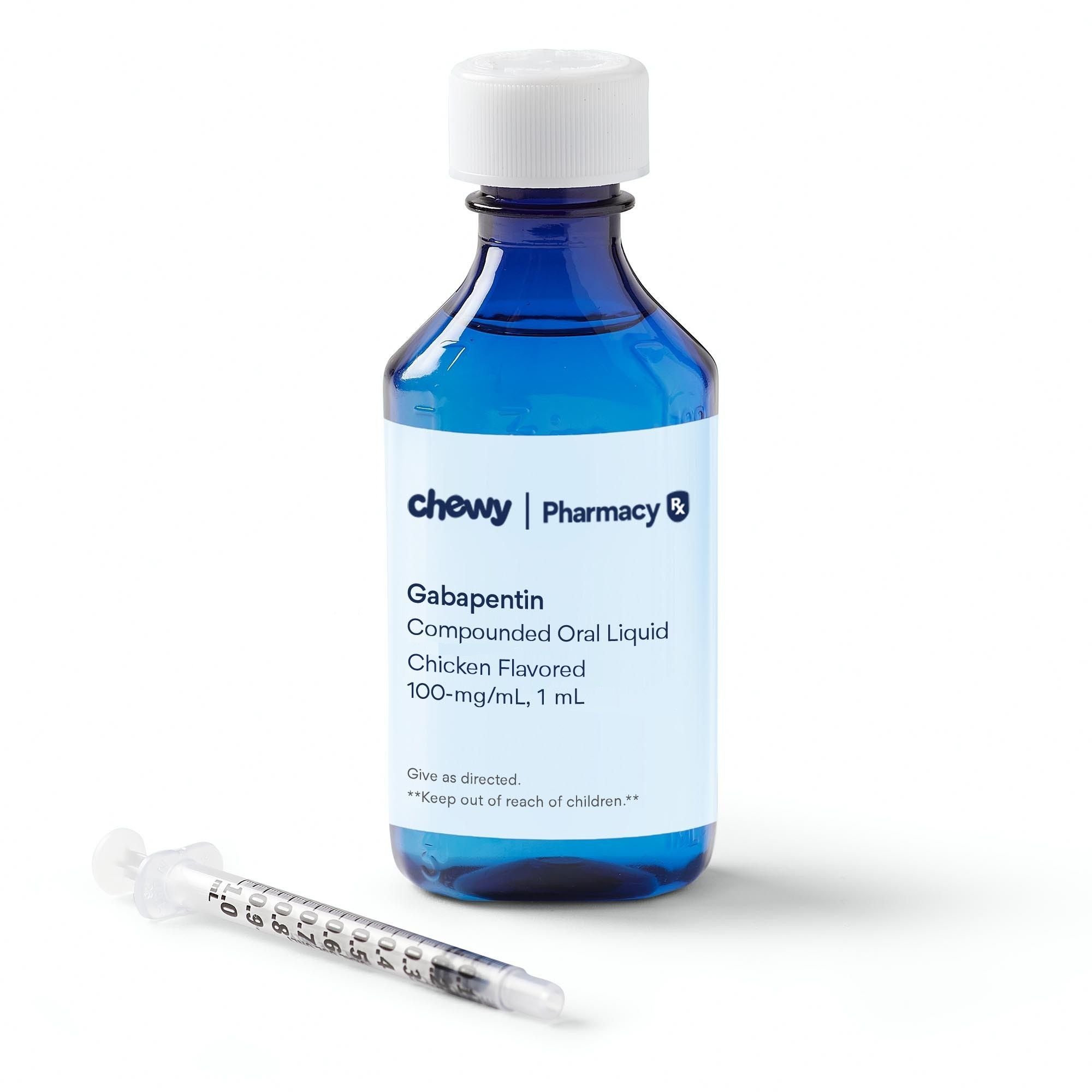
Effect of refrigeration on gabapentin liquid preparations. Gabapentin (100 mg/mL) liquid preparations made from bulk drug powder as well as capsules in Oral Mix and Oral Mix SF was produced as described above. Gabapentin (100 mg/mL) liquid preparations made from capsules in OraBlend (1:1 mixture of OraPlus and OraSweet) and in methylcellulose 1 We then report a comprehensive evaluation of the stability of gabapentin compounded preparations (100 mg/mL) using a validated stability-indicating HPLC-UV method. We studied the effect of the following variables: (1) time: up to 90 days; (2) temperature: 25°C; (3) gabapentin source: bulk drug and capsules; (4) vehicle: Oral Mix and Oral Mix SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Chemical stability of gabapentin in suspension was measured using accurate, reproducible, specific, and stability-indicating high-performance liquid chromatography (HPLC) assay. Gabapentin liquid should be stored in the refrigerator at all times to maintain its stability and potency. Keeping the medication at a consistent temperature is crucial for its effectiveness. However, there may be instances when you need to take the medication with you or have to store it outside of the refrigerator for a period of time. Gabapentin 100 mg/mL oral suspension was prepared using a 1:1 mixture of ORA-Plus®/ORA-Sweet® and stored at both room and refrigerated temperatures. The preparation was prepared using Neurontin capsules 300 mg each and stored in amber plastic prescription bottles. A USP stability-indicating assay method was adapted and optimized for gabapentin analysis. 15 Samples were analyzed with the use of Varian-920 high-performance liquid chromatography with a quaternary gradient pump, autosampler (50 μL sample loop), a UV-Vis detector, and Galaxie chromatographic software. The stationary phase consisted of a Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: carboxymethylcellulose sodium, methylparaben, propylene glycol, propylparaben, purified water, xylitol, anise flavor, artificial strawberry flavor and hydrochloric acid added for adjustment of pH. Gabapentin was measured by accurate, reproducible, specific, and stability-indicating high-performance liquid chromatography (n = 15). The observed mean concentrations exceeded 90% of the initial concentrations in both suspensions for 91 days at 4 degrees C and 56 days at 25 degrees C. Stability of Gabapentin Liquid. Stability refers to the ability of a medication to maintain its safety and efficacy over time, under specified conditions. Gabapentin liquid typically retains its effectiveness when stored at room temperature, but it can degrade due to various factors. Understanding Expiration Dates Gabapentin oral suspension (Neurontin) Refrigerate at 36º to 46º F (2º to 8ºC). Contact manufacturer.*** Off-label § information indicates oral solution stable for seven days at temps up to 86 o F (30 o C). 6 Pfizer 800-438-1935 Glatiramer acetate injection (Copaxone) Refrigerate at 36º to 46º F (2º to 8ºC). One month at room The solution stability of gabapentin in buffered systems was studied in order to facilitate the formulation of a liquid product. The degradation of the drug was followed as a function of pH, buffer concentration, ionic strength, and temperature. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and amber plastic syringes at 25°C / 60%RH for up to 9 The aim of this study is to assess the stability of gabapentin under different stress conditions. This was achieved by using different physical and chemical tests i.e. acid/ base hydrolysis, This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF sus- pending vehicles. We then report a comprehensive evaluation of the stability of gabapentin compounded preparations (100 mg/mL) using a validated stability-indicating HPLC-UV method. We studied the effect of the following variables: (1) time: up to 90 days; (2) temperature: 25°C; (3) gabapentin source: bulk drug and capsules; (4) vehicle: Oral Mix and Oral Mix Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are Understanding Liquid Gabapentin Stability and Storage Requirements. When it comes to liquid gabapentin, understanding its stability is crucial for ensuring its efficacy and safety. Typically, this medication is recommended to be stored in a refrigerator to maintain its integrity. Liquid Gabapentin should never be left out of the fridge for more than 24 hours. After that, it is at risk of becoming contaminated or losing its potency. It also has a short shelf life and must be disposed of after 14 days even if still stored in the refrigerator. Stability: A beyond-use date of up to 56 days at room temperature or 91 days at refrigerated temperature may be used for this preparation. 1,2 Quality Control: Quality-control assessment may include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |