Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
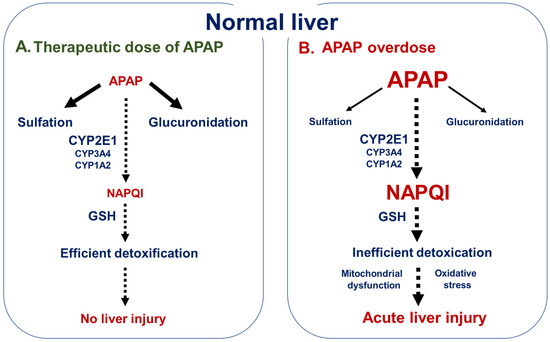
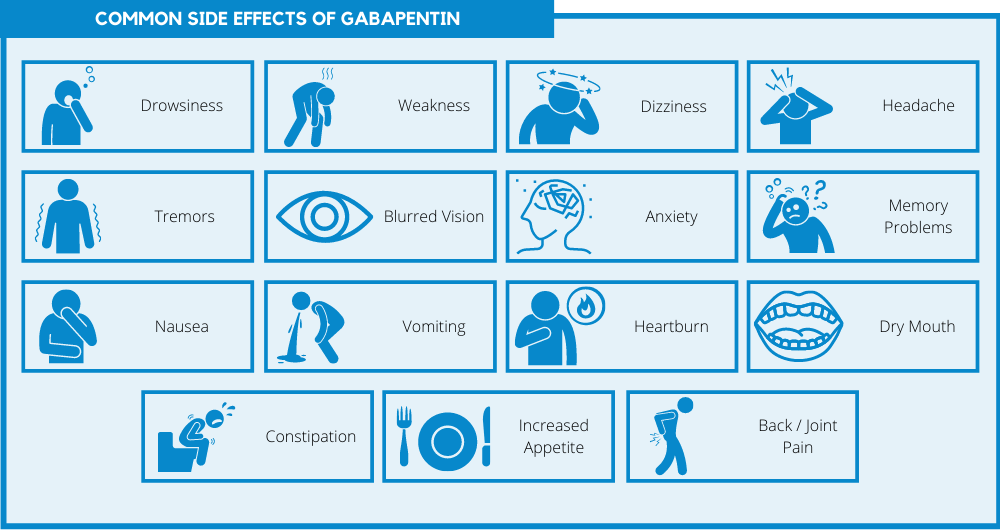
Gabapentin-Induced Liver Toxicity By J. Chahal · 2022 · Cited by 2 — Gabapentin was the sole novel medication introduced prior to the elevation of liver enzymes, and thus was discontinued. On the fifth day, the levels of ALT and AST had Gabapentin is not metabolized by the liver. Instead, it is excreted unchanged in your kidneys after circulating in your blood. Gabapentin affects nerves and chemicals in your body that are involved in some types of pain and in seizures. Many antiretroviral medications have the potential for liver toxicity. But the incidence is much lower with contemporary treatment regimens. The risk of liver damage is higher for people with other liver infections, like hepatitis B, who have alcohol use disorder or who take other medicines that LiverTox® provides regularly updated, unbiased and easily accessed information on the diagnosis, cause, frequency, clinical patterns and management of liver injury attributable to prescription and nonprescription medications and selected herbal and dietary supplements. The LiverTox site is meant as a resource for both physicians and patients as well as for clinical academicians and Gabapentin-Induced Liver Toxicity. Chahal, Japjot MD 1; Arif, Muhammad Osman MD 2; Achufusi, Ted George MD 1. Author Information Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. Gabapentin (Neurontin) usually isn’t bad for your liver or kidneys. In most cases, it has little effect on these organs. In rare instances, gabapentin can cause DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Gabapentin has been implicated in isolated cases of acute liver injury, but in most instances the association was weakened by the coadministration with other potentially hepatotoxic agents. Too few cases have been reported in sufficient detail to characterize the clinical features of the associated injury. Other AEDs with rising and currently highest prescription rates were associated with few or no cases of liver injury including gabapentin (45.3 million), clonazepam (18.8 million), pregabalin (10.6 million), topiramate (9.3 million), and levetiracetam (7.7 million) and many of cases were judged as only “probable”. Klein-Schwartz W, Shepherd JG, Gorman S et al. Characterization of gabapentin overdose using a poison center case series. Journal of Toxicology-Clinical Toxicology 2003; 41(1):11-15. Lofton AL, Klein-Schwartz W. Evaluation of lamotrigine toxicity reported to poison centers. Annals of Pharmaotherapy 2004; 38:1811-1815. Gabapentin should only be given in the tablet or capsule form because the human liquid version of gabapentin contains xylitol which is highly toxic to dogs and can cause liver toxicity and death! Before giving your dog gabapentin, you should mention to your veterinarian any other medications that your dog is currently taking. Limited data are available on the hepatotoxicity of gabapentin. In clinical trials in diabetic neuropathy and epilepsy, therapy with gabapentin was not associated with an increased frequency of serum aminotransferase elevations or liver toxicity. Liver transplantation for drug hepatotoxicity accounted for 15% of liver transplants for acute liver failure over the study period. In our cohort (n = 270), 206 (76%) recipients were female. Am J Ther. 2022 Nov-Dec;29 (6):e751-e752. doi: 10.1097/MJT.0000000000001208. Epub 2020 Jun 5. 1 Internal Medicine, SUNY Upstate Medical University, Syracuse, NY. 2 Gastroenterology, SUNY Upstate Medical University, Syracuse, NY. In two reviews [39, 46] this drug was not associated with severe hepatotoxicity, whereas two case reports of acute liver toxicity induced by gabapentin have been published [47, 48]. In the Italian Gabapentin is an uncommon cause of DILI reported to cause a hepatocellular, cholestatic, or mixed picture of liver injury. Given the limitations of prior cases, we feel our report most closely ties gabapentin use to the resultant transaminase elevation. Gabapentin, a water-soluble amino acid, is eliminated unchanged by the kidneys and there is no appreciable metabolism by the liver. However, there are a few descriptions of gabapentin-related Purpose: Trazodone and gabapentin are commonly used treatments. We report a rare case of trazodone and gabapentin-induced liver injury. Case: A 40-year-old woman with a history of depression presented jaundice. She had no other complaints. The patient denied risk factors for acute and chronic liver disease. Gabapentin lacks liver metabolism; the mechanism by which it produces liver injury is still unknown; however, there are reports of hepatotoxicity associated with its administration, so its use must be individualized for each patient. Herein, we report a gabapentin-induced hepatocellular injury in a patient without another identifiable cause for acute liver injury. Discontinuing gabapentin resulted in rapid reversal improvement in hepatocellular injury. Keywords: drug-induced liver injury; gabapentin; hepatotoxicity.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |